Hello again to those of you who joined my first and second ICP-MS expeditions through the Group 1 and Group 2 elements of the Periodic Table respectively, and a warm welcome to those of you who have just joined me on this journey.
 My ICP-MS expedition through the elements now ventures into the murky and sometimes treacherous territory of the Transition Elements (Groups 3 to 12). I’m expecting this to be an arduous trip, spread across several blog posts, so, if you’re ready, climb on board and buckle up, it could be a bumpy ride.
My ICP-MS expedition through the elements now ventures into the murky and sometimes treacherous territory of the Transition Elements (Groups 3 to 12). I’m expecting this to be an arduous trip, spread across several blog posts, so, if you’re ready, climb on board and buckle up, it could be a bumpy ride.
We’ll begin with Groups 3 and 4, comprising the elements scandium to hafnium. In the interests of brevity for this blog post, I’ll cover the lanthanides and actinides (including lanthanum and actinium themselves) in separate articles.
A short history of the Group 3 and 4 transition elements
You might be wondering why the elements that lie between Groups 2 and 13 are called Transition Elements. This term dates back to a paper published by the English chemist Charles Bury in 1921, in which he stated “During the change of an inner layer from a stable group of 8 (electrons) to one of 18, or from 18 to 32, there occurs a transition series of elements … .” The Transition Elements all are metals, and so they are also often referred to as the Transition Metals.
With the exclusion of lanthanum and actinium, Groups 3 and 4 consist of the stable elements scandium, yttrium, titanium, zirconium and hafnium plus the radioactive element, rutherfordium.
The first member of these two groups to be discovered, by the German chemist Martin Heinrich Klaproth in 1789, was zirconium. Klaproth found zirconium in the form of a new oxide he identified while studying the composition of the mineral jargon (ZrSiO4, also known as jargoon). He named this compound zirkonerde (zirconia), a name which, like jargoon, is believed to derive from the Arabic word zargun meaning ‘gold-hued’ in reference to the colour of the jargon (also known as zircon) minerals in which it is found.
The illustrious English chemist, Sir Humphrey Davy unsuccessfully attempted to isolate zirconium from zircon using electrolysis in 1808 (it was also apparently he who gave this element the name zirconium). In 1824, Jöns Jacob Berzelius, a Swedish chemist, became the first person to isolate zirconium in an impure form by heating potassium hexafluorozirconate (K2ZrF6) with potassium metal. It wasn’t until 1925 that pure zirconium was finally prepared, by the Dutch chemists Anton Eduard van Arkel and Jan Hendrik de Boer through heating zirconium tetrachloride with magnesium, which remains the process by which zirconium is produced today.
The second member of Groups 3 and 4 to be discovered was titanium, identified in 1791 by the British mineralogist Reverend William Gregor during his studies of a sample of ilmeniteore from a stream near the village of Manaccan, Cornwall, UK. From his analysis, he concluded that it was made up of iron oxides and a calx (metallic oxide) of a previously unknown metal. Gregor proposed the name ‘menaccanite’ for the new metal and accordingly reported it to the Royal Geological Society of Cornwall. In 1795, during his investigations of a Hungarian sample of the mineral rutile, Martin Klaproth found that it was the oxide of an unknown element which he named titanium, after the mythical Titans, the 12 children of the primordial gods Uranus (Heaven) and Gaea (Earth).
When Klaproth heard of Gregor’s discovery, he investigated menaccanite and confirmed that it also contained the same element, whereupon he graciously conceded the discovery of titanium to Gregor. The name titanium was adopted as Gregor’s suggestion of menaccanite didn’t allow distinction between the mineral and the metal. It was not until 1910 that titanium was isolated in a pure form by Matthew A. Hunter of the US company General Electric. Hunter developed a process, which still bears his name, of producing titanium by heating titanium tetrachloride with sodium metal at 700 to 800°C.
The next member to be found was yttrium, discovered in the mineral gadolinite by the Finnish chemist Johan Gadolin. In 1789, Gadolin was sent a sample of the mineral by the Swedish geologist and chemist, Carl Axel Arrhenius, who found it in an old quarry close to the village of Ytterby, Sweden in 1787, and originally gave it the name ytterbite. Five years later, in 1794, Gadolin announced that the mineral contained a new metal oxide (later named yttria, after the village of Ytterby) which made up 38% of the sample by weight. The metal itself, named yttrium by convention from the oxide, yttria, was first isolated in 1828 by the German chemist Friedrich Wöhler (who was also the first to isolate beryllium) by reducing yttrium chloride with potassium. As will be revealed in a later expedition, it turned out that yttria was hiding a few more elements).
Scandium was discovered by Lars Frederick Nilson, a Swedish chemist and Professor at Uppsala University, in 1879, during his studies of the minerals euxenite and gadolinite. He and his co-workers were actually looking for rare earth metals at the time. By processing euxenite and other residues of rare-earth minerals, Nilson was able to prepare about 2 g of scandium oxide (scandia, Sc2O3) of high purity. It was Nilson who named the new oxide scandia, after the Latin name for his native Scandinavia, and the metal therein hence became known as scandium. Interestingly, back in 1871 the father of the Periodic Table, Dmitri Mendeleev had predicted that an element should exist that would resemble boron in its properties. He called this hypothetical element ekaboron and gave it the symbol Eb. It was the Swedish mineralogist, Per Theodor Cleve who, shortly after the discovery of scandium proved that this new element was Mendeleev’s predicted ekaboron. Metallic scandium was produced for the first time in 1937 by electrolysis of a mixture of potassium ,lithium, and scandium chlorides, at 700 to 800°C, by the German chemists Werner Fischer, Karl Brünger and Hans Grienseisen at the Albert Ludwigs University in Freiburg, Germany.
Like scandium, the existence of the element that became known as hafnium was predicted by Dmitri Mendeleev. His 1869 Periodic Table had predicted the existence of an element that was heavier than titanium and zirconium, but with similar chemical properties. Mendeleev initially placed lanthanum in this position, but upon its eventual discovery after his death, hafnium slotted correctly into place, thereby validating his original prediction. The story of hafnium’s actual discovery began in 1911 when the French chemist Georges Urbain claimed to have isolated a new element, which he named ‘celtium’, from a sample of rare earth element residues.
His work was interrupted by World War 1, so it was not until 1922 that he was able to announce his discovery. Through his characterisation of what he believed to be the emission spectrum of his new element, he mistakenly identified it as a rare earth. In 1923, the Hungarian radiochemist, George de Hevesy, together with the Dutch physicist, Dirk Coster, while carrying out X-ray spectroscopic analysis of a zirconium ore, confirmed that the new element was not in fact a rare earth, but rather a member of the same group as zirconium.
As they made their discovery at the Niels Bohr Institute of the University of Copenhagen, they elected to name the new element hafnium after ‘Hafnia’, the Latin name for Copenhagen. Although Urbain was right about having detected the presence of a new element, the spectra and the chemical behaviour he described were not a good match to the element identified by de Hevesy and Coster and so they were ultimately credited with hafnium’s discovery.
Hafnium is notoriously difficult to separate from zirconium, in whose ores it is principally found. Valdemar Thal Jantzen and de Hevesey successfully separated it from zirconium as hafnium fluoride through repeated recrystallization of the double ammonium an dpotassium fluorides, but it was the Dutch chemists Anton Eduard van Arkel and Jan Hendrik de Boer, who were the first to prepare metallic hafnium by passing hafnium tetraiodide vapor over a heatedtungstenfilament in 1924. This process for differential purification of zirconium and hafnium is still in use today.
The final member of Groups 3 and 4, rutherfordium, was first identified by Georgy Flerov and colleagues at the Dubna Nuclear Institute, Russia, in 1964 and later by Albert Ghiorso’s team at the University of California, Berkeley, USA. The Russian group originally named the element, which they detected at mass 259 following high energy collision reactions between242Pu and22Ne ions, as kurchatovium (Ku), in honour of Igor Vasilevich Kurchatov, the late Head of Soviet Nuclear Research. Ghiorso’s team, who used collisions between249Cf and12C to create 257Rf and 258Rf, proposed the name rutherfordium (Rf) after the eminent New Zealand physicist, Ernest Rutherford. A dispute over priority of discovery followed and eventually, in 1992, the International Unions of Pure and Applied Chemistry (IUPAC) concluded that both the Russian and American researchers had been justified in making their claims. Today, rutherfordium is the preferred IUPAC name.
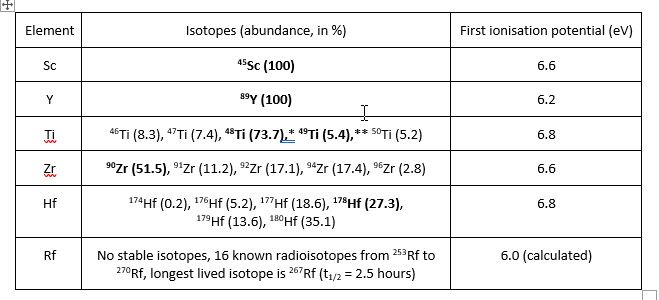
Table 1. Key properties for the Group 3 and 4 elements for ICP-MS analysis
(preferred isotopes for analysis in bold).
48Ti is preferred when using triple quadrupole ICP-MS
** 49Ti is generally preferred when using single quadrupole ICP-MS
As with all groups in the Periodic Table, the relative signal sensitivity (i.e., corrected for isotope abundance) of the Group 3 and 4 elements in ICP-MS varies with mass, primarily as a result of space charge repulsion in the ICP-MS interface region which reduces the transmission of lighter ions compared to heavier ones. In spite of this, the Group 3 and 4 elements still have the potential to be detected down to the parts per trillion detection (ppt or μg L-1) level, but unlike the lighter mass members of Groups 1 and 2, the lighter Group 3 and 4 elements lie in an interference infested region of the ICP-MS mass spectrum which affects their achievable limits of detection. In the next part of this blog, I’ll briefly discuss how to resolve these interference problems and will address other issues encountered while measuring the Group 3 and 4 transition elements using ICP-MS.
Interferences and other considerations for Group 3 and 4 element analysis by ICP-MS
Scandium, mono-isotopic at mass 45, is not often an important element for analysis and is usually absent or only present at low levels in most sample types. For these reasons, it’s a popular choice for a lower mass internal standard. It is most significantly interfered by 12C16O21H+, but this can be easily removed using either single or triple quadrupole instruments operated in He KED collision cell mode.
In solutions containing high levels of calcium, 44Ca1H+ can potentially be a problem, but as Sc is usually present as an internal standard, this can be overcome by simply increasing the Sc concentration to a level where the effect of CaH on the Sc signal is negligible. Interference from 29Si16O+ is also a possibility, but this can be resolved using a triple quadrupole instrument operated in oxygen mass shift mode.
Finally, in high concentration Zr solutions, 90Zr2+ can interfere with Sc, but this is a rare occurrence and like SiO, can be resolved using a triple quad system or a high-resolution ICP-MS instrument. Scandium washes out reasonably quickly using 2% (v/v) HNO3 as the wash solution and is not usually an element that has contamination problems.
Yttrium, also mono-isotopic, at mass 89 is generally not significantly interfered and, like Sc, is a popular internal standard element as it is usually not present at significant levels (with the exception of certain geological samples). One potential interference is 88Sr1H+, which may be resolvable using a triple quad method (I haven’t tried this yet) or, alternatively, using mathematical correction. Yttrium washes out easily with 2% (v/v) HNO3 and does not usually suffer with contamination.
Titanium is more problematic in terms of interferences. The most significant are 48Ca, 31P16O+, 32S16O+, 14N16O2+ and 50Cr. The polyatomic interferences can be efficiently suppressed using single quadrupole ICP-MS with KED operation in most cases, but in samples where P and S are very high, triple quadrupole (with NH3 as the cell gas and mass shift operation) or high-resolution instrumentation is required. To resolve the isobaric 48Ca and 50Cr interferences on 48Ti and 50Ti, triple quadrupole operation with NH3 cell gas and mass shift to selected Ti-NH3 cluster ions is again required. Titanium can be a little bit sticky in the sample introduction system but usually, a combined 2% (v/v) HNO3 and 2% (v/v) HCl acid rinse is sufficiently effective.
Zirconium is quite easy to measure as its most abundant isotope, 90Zr is largely interference-free in the majority of analytical situations. It is fairly easy to wash out with HNO3, although an HCl/HNO3 mixture may be required when measuring samples containing varying levels of Zr.
Finally, hafnium is straightforward to measure with no interference problems on its second most abundant isotope (178Hf). It’s most abundant isotope (180Hf) is overlapped by W and Ta isotopes, which although both low in abundance, could cause false-positive results for Hf. Hafnium is similar in washout behaviour to both Ti and Zr, so a mixed acid rinse also helps reduce sample to sample carryover. I should also mention that all Group 4 elements benefit from the presence of trace amounts of hydrofluoric (HF) acid to improve their longer-term stability in solution. If you choose to use HF though, it is of course essential to handle this hazardous acid with great care: wear gloves and safety glasses, and in the event of coming into direct contact with it, immediately wash the affected area thoroughly with water and apply calcium gluconate gel.
And so, at last, our third expedition through the elements comes to an end. Join me soon on my next trip, on which we will head deeper into the realm of the transition group elements and uncover the secrets of the elements of Groups 5 and 6.
References and further reading
1 Langmuir’s Theory of the Arrangement of Electrons in Atoms and Molecules, Charles R. Bury, Journal of the American Chemical Society, Vol. 43, p. 1602-1609, 1921.
To learn more about the history of the Periodic Table and the properties of the elements, head to the following websites:
https://www.rsc.org/periodic-table
https://www.webelements.com/
https://en.wikipedia.org/wiki/Periodic_table
Additional Resources
To explore our range of AA, ICP-OES and ICP-MS instruments, head to our trace elemental analysis instrumentation page.
Visit our Food and Beverage, Pharmaceutical and Environmental pages to learn about how our trace elemental analysis solutions can meet your analytical needs.
Subscribe to one of our Community pages to only receive e-mails relating to the application area that’s most relevant to you.
An ICP-MS Expedition through the Elements – Part 1
An ICP-MS Expedition through the Elements – Part 2
An ICP-MS Expedition through the Elements – Part 4