Hello once again to those of you who joined my previous ICP-MS expeditions through Group 1, Group 2 and Groups 3 and 4 of the Periodic Table, and welcome to those of you who have only just joined me on my travels.
 My ICP-MS expedition through the elements now pushes on further into the depths of Transition Elements territory. This next phase of the trip tackles Groups 5 and 6 of the Periodic Table, a region where interferences abound and analytical signals are often not what they appear to be. Getting the right results from your ICP-MS in this part of the Periodic Table is a tricky business if you’re not prepared. So, if you’re ready, saddle up and let’s set off on this challenging leg of the journey.
My ICP-MS expedition through the elements now pushes on further into the depths of Transition Elements territory. This next phase of the trip tackles Groups 5 and 6 of the Periodic Table, a region where interferences abound and analytical signals are often not what they appear to be. Getting the right results from your ICP-MS in this part of the Periodic Table is a tricky business if you’re not prepared. So, if you’re ready, saddle up and let’s set off on this challenging leg of the journey.
History of the Group 5 and 6 Transition Elements
The Group 5 and 6 elements are chromium (Cr), molybdenum (Mo), tungsten (W), dubnium (Db), vanadium (V), niobium (Nb), tantalum (Ta) and seaborgium (Sg).
The first of these to be identified was molybdenum, in 1778, by the German-Swedish chemist, Carl Wilhelm Scheele. While studying an ore known as molybdenite, Scheele concluded that it did not contain lead as many others had suspected at the time and reported that it instead contained a new element which he was unsuccessful in extracting.
Scheele sent a sample of an oxide of his suspected new element, which he had obtained through acid treatment of molybdenite, to the Swedish chemist Peter Jacob Hjelm, who managed to obtain the first sample of the impure metallic element by grinding the oxide into a paste with carbon and linseed oil and heating it to reduce the oxide. Hjelm announced the discovery of Scheele’s elusive element in 1781 and named it molybdenum, a name that actually derives from the Ancient Greek word “molybdos” meaning “lead,” reflecting the belief that molybdenite was a lead-bearing mineral.
The second member to be found was tungsten. Its existence was first deduced in 1779 by the Irish chemist Peter Woulfe from his analysis of the mineral wolframite. In 1781, it was Carl Wilhelm Scheele who isolated tungsten as hydrated tungstic oxide (WO3.H2O, also referred to as tungstic acid) from the mineral scheelite, but once again he was unable to reduce this oxide to the metal as he didn’t have a suitable furnace.
As a brief aside here, Scheele was a most unfortunate chemist. During his career, he is known to have discovered the existence of at least six other elements (oxygen, barium, chlorine, manganese, nitrogen and oxygen) but due to a combination of publishing his results too late and/or not quite being able to isolate the elements he found in their elemental state, he didn’t get recognised as the discoverer. As a result, the science fiction author, Isaac Asimov, famously awarded him the nickname “Hard Luck Scheele” (posthumously, since Asimov was born 114 years after Scheele died).
Tungsten was finally isolated by two Spanish brothers, Fausto and Juan Jose de Elhuyar, in 1783, in Bergara, Spain, by reduction of acidified wolframite with charcoal. They originally named it wolfram after the mineral from which they extracted it (hence its chemical symbol, W). The name “tungsten,” which means “heavy stone” in Swedish and was the original name given to the mineral scheelite, is used in English, French, and several other languages, but, curiously, not in the Nordic countries, where the name “wolfram” is used. “Wolfram” (or occasionally “volfram”) is also the name used in most European countries.
Chromium was the next to be discovered, by the French chemist Louis-Nicholas Vauquelin in 1797. During the mid-18th century, analysis of the mineral crocoite (also known as Siberian “red lead” and discovered in 1761 by the German mineralogist, Johann Gottlob Lehmann, in the Beryozovskoye mines in the Ural mountains) showed that together with lead, another material was present. In 1794, Vauquelin received some samples of crocoite ore, his analysis of which confirmed that it was a lead mineral.
He subsequently dissolved it in hydrochloric acid, precipitated the lead, filtered this off and then shifted his attention to the remaining liquor, from which he succeeded in isolating chromium as chromium trioxide. Vauquelin was intrigued by the range of colours that his newly discovered substance could produce in solution, and so he named it chromium from the Greek word “chroma” meaning “colour.” In 1797, Vauquelin was able to finally isolate metallic chromium by heating chromium trioxide together with charcoal in an oven. Incidentally, it was also Vauquelin who discovered that the green colour of emeralds is largely caused by chromium, through his studies of a Peruvian emerald sample.
Four years later, in 1801, niobium was discovered by the British mineralogist Charles Hatchett. While examining minerals in the British Museum, Hatchett’s attention was caught by a sample labeled columbite. From his studies of it, he suspected that it contained a new metal, and it transpired that he was right. Through heating a sample of columbite with potassium carbonate, then dissolving the product in water and acidifying the solution, he obtained a precipitate.
Despite his efforts, he was not able to isolate the element itself from this precipitate, but he nonetheless christened the new element he was convinced the precipitate contained columbium, a name which persisted for several years. What followed was a period of controversy during which the existence of Hatchett’s columbium was disputed, driven by its very similar properties to tantalum, which was discovered shortly after columbium, in 1802 (more on tantalum shortly).
In 1809, English chemist William Hyde Wollaston compared samples of the oxides derived from both columbium (columbite) and tantalum (tantalite) and concluded that the two oxides were identical, despite them having significantly different densities. He believed that columbium and tantalum were one and the same, and declared that only tantalum existed. This conclusion was disputed in 1846 by the German chemist Heinrich Rose, who concluded that there were two different elements in the tantalite sample. Rose gave the two elements the names niobium and pelopium, after the children of the Greek mythological king Tantalus, Niobe and Pelops.
The situation became more confused through claims by the German chemist R. Hermann and the German mineralogist Wolfgang von Kobell that there were two other elements existing alongside niobium and tantalum, which they named ilmenium and dianium respectively. The differences between tantalum and niobium were eventually unequivocally demonstrated in 1864 by the Swedish mineralogist and chemist Christian Wilhelm Blomstrand who, together with a number of others, proved that there were only two elements.
In 1864, Blomstrand became the first person to isolate niobium metal when he reduced niobium chloride by heating it in an atmosphere of hydrogen. As mentioned above, columbium (symbol Cb) was Charles Hatchett’s original choice of name for niobium, a name that reflected the provenance of the ore in which he identified it (i.e., Columbia, the historical name for America). This name remained in use in American journals until 1953, while niobium was used in Europe. To end the confusion, the name niobium was chosen for element 41 at the 15th Conference of the Union of Chemistry in Amsterdam in 1949 and officially adopted a year later.
Vanadium was also discovered in 1801 …. well, almost. In fact, this element has the notable history of being discovered twice, although unfortunately for the Spanish-Mexican scientist Andres Manuel del Rio who first identified it as a new element (which he named erythronium) in a mineral now known as vanadinite, the discovery was thrown off course when he sent a sample to Paris where it was erroneously concluded by the French chemist Hippolyte Victor Collet-Descotils that it was a chromium mineral.
The second time vanadium was discovered was not until 30 years later, in 1831, by the Swedish chemist Nil Gabriel Sefstrom at Stockholm. He separated it (as chlorides of vanadium) from a sample of cast iron made from ore that had been mined in the province of Smaland, in the south of Sweden, and was able to show that it was a new element.
In so doing, he beat a rival scientist, the German chemist Friedrich Wohler who was studying a vanadium mineral from the Mexican town of Zimapan, to the discovery. Sefstrom named the element vanadium after the old Norse name for the Scandinavian goddess of beauty and fertility, Vanadis, a name inspired by the beautiful colours of vanadium’s compounds in solution. It was the English chemist Henry Enfield Roscoe who first isolated vanadium metal in 1867 by reducing vanadium dichloride with hydrogen.
As briefly mentioned above, tantalum was discovered in 1802, by the Swedish analytical chemist Anders Ekeberg during his analysis of two mineral samples, yttrotantalite from Ytterby, Sweden and tantalite from Kimito, Finland. Ekeberg was, from childhood, an avid fan of Greek literature and because it was such a tantalising task to confirm the existence of this new element, he named it tantalum in homage to the Greek character, King Tantalus. As mentioned earlier, it was Heinrich Rose who eventually confirmed that niobium and tantalum were two separate elements.
The matter was finally settled with certainty when the Swiss chemist Jean Charles Galissard de Marignac managed to separate them as a result of some arduous chemistry that resulted in his discovery of the insolubility of potassium heptafluotantalate compared to potassium oxypentafluoroniobate monohydrate in 1866. Marignac’s method was used for separating tantalum and niobium for decades, but today his method has been replaced by solvent extraction from fluoride containing tantalum /niobium solutions.
The unstable, radioactive members of Groups 5 and 6, dubnium and seaborgium, were discovered in between 1968 and 1974. In 1968, Georgy Flerov’s team at the Russian Joint Institute for Nuclear Research (JINR) produced an isotope with atomic number 105 through bombardment of americium (249Am) with a beam of neon ions (22Ne+).
After further experimentation to cement their claim to its discovery, Flerov’s team named the element nielsbohrium (Ns) in honour of the Danish nuclear physicist Niels Bohr. In April 1970, a team led by Albert Ghiorso at the Lawrence Berkeley Laboratory (LBL), in Berkeley, California claimed to have synthesized element 105 by bombarding californium (243Cf) with nitrogen ions (15N+). They named it hahnium (Ha) after the German chemist Otto Hahn, widely regarded as the father of nuclear chemistry.
The Russian team’s delay in naming their new-found element led the US team to conclude that they had insufficient data to confirm their discovery, which lead to a long period of heated controversy over discovery and naming rights for element 105. After many disagreements and numerous name changes, finally, in 1996, IUPAC decreed that element 105 should be named dubnium (Db), after the Russian town of Dubna, the location of the JINR.
The discovery of the final member of Groups 5 and 6, element 106, seaborgium, was also subject to controversy. Evidence of element 106 was first reported in 1974 by a Russian team at the JINR in Dubna led by Yuri Oganessian. His team claimed to have discovered this element after bombarding lead (207Pb and 208Pb) targets with accelerated chromium ions (54Cr+).
A few months later, researchers including Glenn T. Seaborg, Carol Alonso and Albert Ghiorso at the University of California, Berkeley, and E. Kenneth Hulet from the Lawrence Livermore National Laboratory, also claimed to have synthesized element 106 by bombarding a californium (249Cf) target with oxygen ions (18O+). A dispute, therefore, arose from the initial competing claims of discovery, a dispute that lasted until 1992, when the IUPAC/IUPAP Transfermium Working Group (TWG), concluded that the Russian synthesis of seaborgium-260 did not have sufficiently convincing data, whereas the American synthesis of seaborgium-263 was convincing on the basis of its connection to known daughter nuclei.
The TWG subsequently recognised the Berkeley team as the official discoverers of element 106 in their 1993 report. After being recognized as official discoverers, the Berkeley team set about naming the element and after considering names derived from various famous scientists, eventually chose to name it after Seaborg himself. The name seaborgium and symbol Sg were announced at the 207th National Meeting of the American Chemical Society in March 1994, but the controversy did not end there as IUPAC ruled in August 1994 that an element could not be named after a living person. The worldwide furor that erupted as a result of this decision, which infringed on the right of an element’s discoverers to name their element, led to IUPAC reinstating seaborgium as the name of element 106 in August 1995. The matter was ultimately fully resolved when Seaborg passed away in 1999 at the age of 86.
Table 1 shows some key properties of the Group 5 and 6 elements with respect to their analysis by ICP-MS.
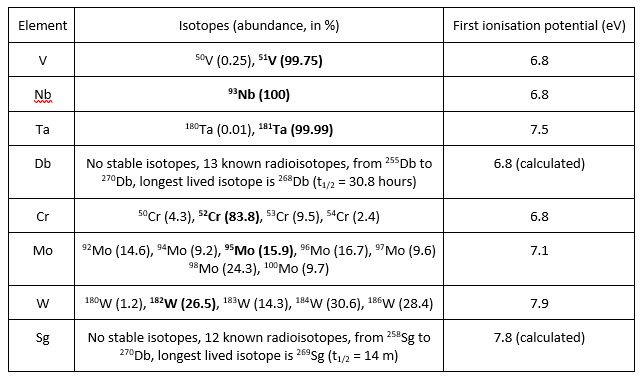
Table 1. Key properties for the Group 5 and 6 elements for ICP-MS analysis
(preferred isotope for analysis in bold).
Interferences and other considerations for Group 5 and 6 element analysis by ICP-MS.
Vanadium is almost mono-isotopic, given that its main isotope, 51V, has an abundance of > 99%. Its most significant interference is 35Cl16O+, which is commonly encountered because chlorine (Cl) is present in many different sample types in the form of chloride. Usually, the signal contribution from ClO+ is quite small and can be simply removed using single quadrupole ICP-MS operated in He KED collision cell mode. In samples containing high levels of chlorine, such as HCl solutions, triple quadrupole ICP-MS using either NH3 (for on-mass interference removal) or O2 (for mass-shift interference removal) cell gas is required to efficiently remove ClO+. High levels of sulphur can sometimes be an issue for vanadium analysis because of various SOHx+ interferences but these are easily removed using triple quadrupole ICP-MS technology or using a high-resolution ICP-MS instrument. Vanadium washes out reasonably well using a 2% (v/v) HNO3 wash and is very rarely an element that suffers from contamination problems.
Niobium, mono-isotopic at mass 93, is not significantly interfered for most applications. With specialised applications such as trace analysis of niobium in the presence of high levels of zirconium or molybdenum, interference from 92Zr1H+ and 92Mo1H+ can arise. In these situations, triple quadrupole ICP-MS provides an effective solution through mass-shift reaction of Nb with O2 cell gas. Niobium washes out quite well with just nitric acid, but with respect to longer-term stability in solution, it can benefit from the presence of trace amounts of hydrofluoric acid (HF). As I mentioned in part 3 of this blog post series, if you use HF, it is, of course, essential to handle this acid with great care — wear gloves and safety glasses, and in the event of coming into contact with it, immediately wash the affected area thoroughly with water and apply calcium gluconate gel to said affected area.
Tantalum, like vanadium, is almost mono-isotopic, with most of it (> 99.9%) being present at mass 181. Being a high mass element, tantalum is rarely interfered, but can be subject to interference in the presence of high levels of hafnium (from 180Hf1H+), tungsten (from 180W1H+) and potentially from 165Ho16O+ if you happen to be trying to measure Ta at trace levels in the presence of Hf, W or Ho. Tantalum reacts efficiently with O2 so the aforementioned interferences could potentially be resolved using triple quadrupole ICP-MS, but I have not explored this as yet.
Like niobium, tantalum is more stable in solution in the presence of trace amounts of HF and will also wash out more effectively in the presence of a low (< 0.05% (v/v)) level of HF acid. It should be noted that if you have a need to improve washout of Nb and/or Ta and you plan to add trace HF to your samples, standards and rinse solution, you might need to consider using an inert sample introduction system rather than the conventional quartz nebuliser and spray chamber, as HF degrades quartz.
Chromium suffers from multiple interferences, as its isotopes lie in the most interference-infested region of the ICP-MS mass spectrum. The main interferences are 36Ar16O+, 40Ar12C+, 38Ar14N+, 35Cl16O1H+ and 37Cl16O+. All these interferences are quite easily removed using single quadrupole ICP-MS with collision cell operation and pure helium as the cell gas. High-resolution ICP-MS is also very effective for resolving the common interferences on chromium and if you are battling very high chloride-based interferences on this element, triple quadrupole ICP-MS with a mass-shift reaction of Cr with O2 cell gas also works well.
Chromium washes out easily with dilute nitric acid but can sometimes suffer from contamination, so pre-cleaning labware is recommended if you are planning to measure it at trace levels. For more advice on solving and/or minimising contamination problems, take a look at my blog post on this subject.
Molybdenum, positioned at higher mass than chromium, is much less interfered than the latter and can generally be measured in standard mode with no cell gas being required. Molybdenum has direct isotope overlaps with zirconium and ruthenium but these can be avoided through selection of non-interfered Mo isotopes. There are some selenium oxides than can potentially interfere with molybdenum, such as 80Se16O+, but these are rarely a problem as selenium is not usually present at high concentrations in most samples. Molybdenum can be a little sticky in the ICP-MS sample introduction system, but this can be solved by addition of HCl (at 0.5% (v/v)) to the rinse solution.
Finally, tungsten is, to all practical intents and purposes not interfered. It does have some direct isotope overlaps with tantalum and osmium, but neither of these are encountered at high concentration in the vast majority of samples, so are unlikely to present a problem. Tungsten does suffer with washout and memory effects in the sample introduction system, but if low-level tungsten analysis is required, washout can be improved by addition of HF acid to a concentration of 0.05% (v/v).
With that, our fourth ICP-MS expedition through the elements comes to an end. Join me in a few weeks on my next excursion, which will take us ever onwards into Groups 7 and 8 in the heart of the transition metals.
Further Reading
If you would like to learn more about the history of the discovery of the elements and their properties, head to the following websites:
Discovery of the Elements, Mary Elvira Weeks, 6th Edition, 1960 – https://archive.org/details/discoveryoftheel002045mbp/mode/2up
Interactive Periodic Tables: https://www.rsc.org/periodic-table and https://www.webelements.com/
General Periodic Table information – https://en.wikipedia.org/wiki/Periodic_table
Additional Resources
An ICP-MS Expedition through the Elements — Part 1
An ICP-MS Expedition through the Elements — Part 2
An ICP-MS Expedition through the Elements — Part 3
To explore our range of AA, ICP-OES and ICP-MS instruments, head to our trace elemental analysis instrumentation page.
Visit our Food and Beverage, Pharmaceutical and Environmental pages to learn about how our trace elemental analysis solutions can meet your analytical needs.
Subscribe to one of our Community pages to only receive e-mails relating to the application area that’s most relevant to you.