Novel drug development in the fight against the HIV pandemic
Despite incredible advances in treatment, there is still no cure or vaccine for human immunodeficiency virus (HIV), and the disease persists as a global pandemic to this day.
According to the World Health Organization, the number of people living with HIV has risen to 39.9 million worldwide (as of 2023), with 1.3 million people acquiring HIV in the past year alone. Additionally, an estimated 630,000 deaths have been attributed to acquired immunodeficiency syndrome (AIDS) and other HIV-related causes in 2023. Most available HIV drugs require continuous administration to suppress the expression of the virus, making them an impractical and inaccessible solution in many developing nations where the disease is spreading most aggressively.
It is evident that novel treatments for HIV continue to be of paramount importance. For over 30 years, structure-based drug design has played a vital role in the development of HIV treatments. By visualizing key viral proteins in 3D, researchers have developed highly specific and targeted compounds, such as protease inhibitors, that suppress the ability of the virus to replicate. While these drugs have been successful in neutralizing the virus within infected individuals, a vaccine that prevents infection altogether has remained elusive.
New research, using cryo-electron microscopy (cryo-EM) for structural analysis, has made significant strides in the search for neutralizing antibodies that can target multiple HIV strains. Understanding their mechanisms serves as the first step toward a potential vaccine that elicits a broad, preventative antibody response to HIV infection.
Targeting broadly neutralizing HIV-1 antibodies
Broadly neutralizing antibodies (bnAbs) are essential for a practical HIV vaccine, as there is a wide, diverse range of HIV variants that the body’s immune system must be ready to respond to. In humans living with HIV, bnAbs only develop rarely, and this process can take years, which is why vaccination routines attempt to accelerate and guide their natural development.
A recent study led by researchers at The Scripps Research Institute, La Jolla, utilized a vaccine design approach called germline targeting to create bnAbs in primates containing heavy chain complementarity determining region 3 (HCDR3), which is a structure typically necessary for antibody recognition of HIV. Germline targeting first primes B cells that produce antibodies with the structures necessary for a bnAb (such as the HCDR3) and then follows up with a series of immunogens that further guide the development of the antibody.
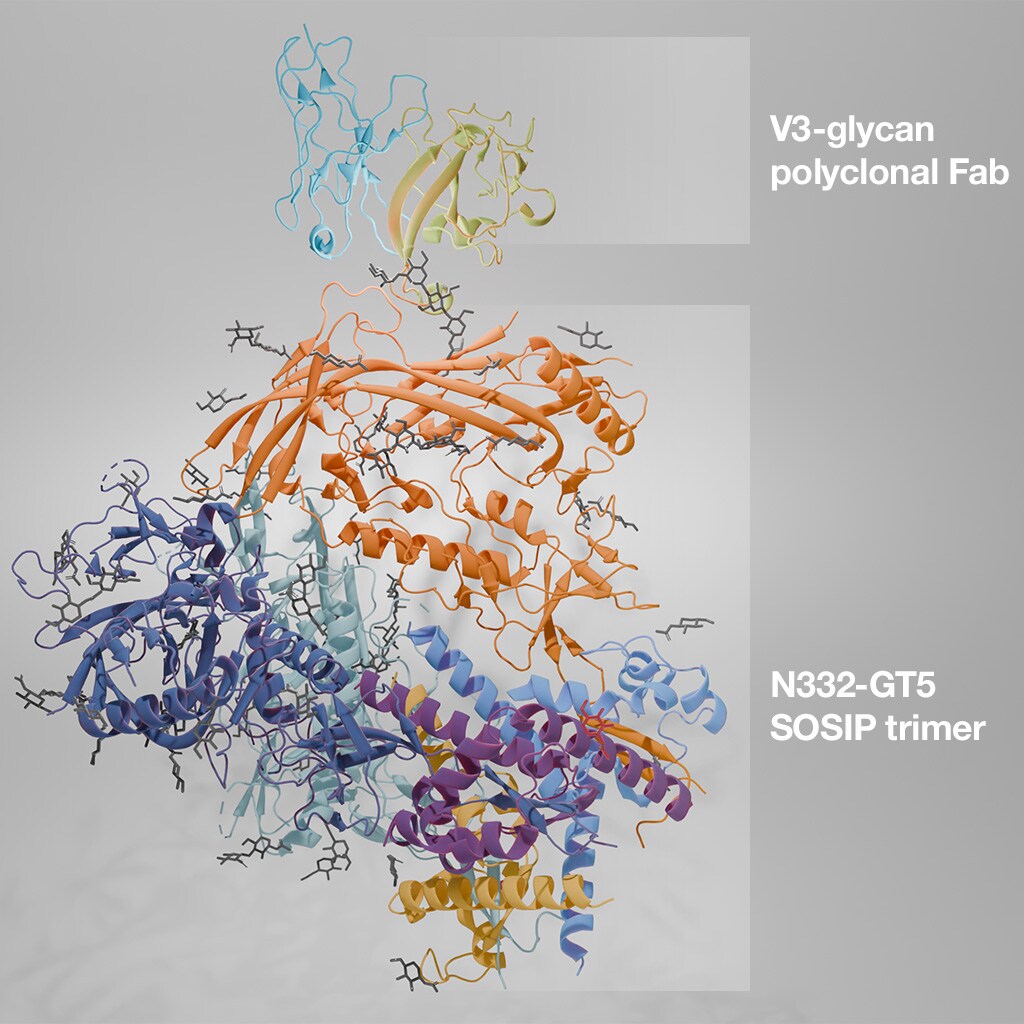
Cryo-EM structure of an HIV SOSIP trimer in complex with a polyclonal Fab, isolated from immunized mice. PDB ID: 8F9G.
Using cryo-EM, they were able to visualize the resulting HCDR3-containing bnAb structures, as well as their binding to HIV trimer glycoproteins. This allowed them to confirm the behavior and interaction of the resulting antibodies, and provides a proof of concept for a similar approach in humans.
HIV vaccine regimens produce mature bnAbs in humans
A follow-up study utilized the germline targeting approach in combination with mRNA vaccination strategies to develop a course of boost immunogens that enhance the maturation of the bnAbs. They found that a regiment of multiple boost immunogens, delivered via mRNA lipid nanoparticles, greatly enhanced the affinity of the resulting bnAbs and allowed for the refinement of bnAb development, even after the antibody precursors had begun to mature in germinal centers.
Research collaborations between Scripps, MIT, and the NIH are continuing to explore vaccine regimens as a means to mature precursor antibodies into bnAbs, finding success in the use of nanoparticle immunogens in mice. Cryo-EM assisted in the structure-based development of the immunogens used in these studies.
Clinical trials of HIV vaccines in humans
Translating the previously-mentioned germline targeting approach to clinical trials, researchers at the Duke School of Medicine have tested an immunogen vaccine consisting of a peptide-liposome that targets an external portion of the HIV envelope glycoprotein (MPER), which is a conserved region near the viral membrane. One of the key aspects of their vaccine treatment was guiding the antibody toward otherwise improbable mutations, which were essential in enhancing its neutralizing capabilities. The most potent bnAbs resulting from their approach were able to “neutralized 15% of global tier 2 HIV-1 strains and 35% of clade B strains … after the second immunization.” Their clinical trial results provide a highly promising framework for efficient and rapid HIV vaccine development.
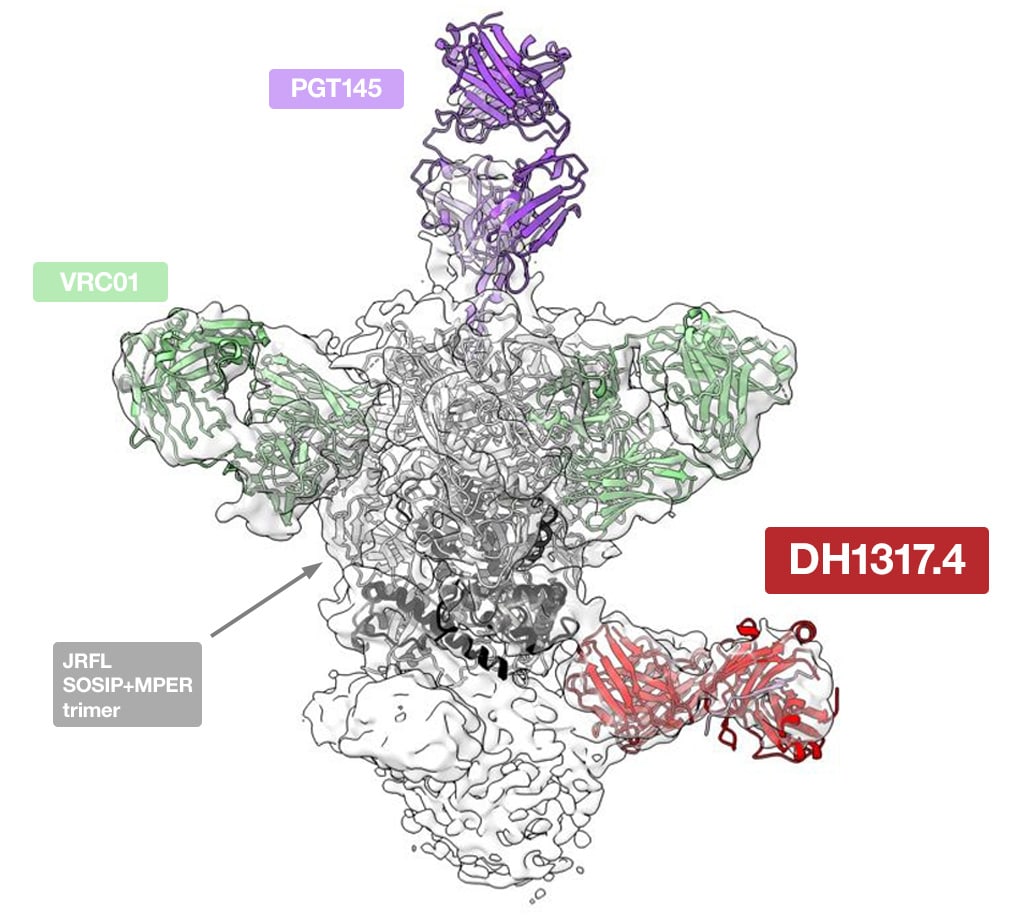
Cryo-EM structure of the human DH1317.4 antibody, elicited by vaccination, bound to the full-length HIV-1 Env JRFL SOSIP trimer and stabilized with PGT145 and VRC01 Fabs. Image kindly provided by Prof. Priyamvada Acharya and Prof. Wilton B. Williams. doi: 10.17632/fc9ps8rhn5.1
Cryo-EM was a critical aspect of their investigation; single particle analysis was used to clearly visualize the interaction of neutralizing antibody lineages with the envelope antigen. By understanding these mechanisms at a molecular level, they were able to rationally tune the behavior of the immunogen to optimize the antibody response.
Conclusions
While there is still no broadly effective HIV vaccine, structure-based methods for immunogen design have made great strides towards potential vaccine regimens that can prepare our bodies to fight this challenging disease. Molecular insights are critical to this rational design approach, and cryo-electron microscopy is playing a vital role in the visualization and contextualization of antigen-antibody interactions.
Learn more about cryo-EM in rational drug design
Tilak Gupta is a Product Marketing Manager at Thermo Fisher Scientific.
Written in collaboration with Alex Ilitchev, Science Writer, Thermo Fisher Scientific and Benice van Gerven, Market Development Specialist, Thermo Fisher Scientific.

Leave a Reply