Analgesics, pain relief, and opioid addiction
While pain management is a critical and highly complex aspect of modern medicine, pain-relieving substances (or analgesics) are also some of the earliest known therapeutics in recorded human history. Opium, derived from certain species of poppies, is perhaps one of the first analgesics, and it gives its name to both a class of drugs (opioids) as well as their targets, opioid receptors. Unfortunately, the history of these medicines is highly intertwined with their addictive and destructive properties. Even to this day, opioids prescribed for severe pain carry a significant risk of addiction and their administration must be closely regulated.
The over-prescription and over-use of these opioid analgesics (also called narcotics) has led to a global opioid crisis, with ~60 million people addicted to opioids, resulting in ~100,000 deaths by opioid overdose annually. Pharmaceutical research is therefore continuously striving to develop novel analgesics that are highly effective, targeted, and safer, with significantly reduced risks of addiction.
To develop these drugs, researchers are finding it increasingly necessary to understand the interaction of opioids and their receptors at a molecular level. Leading structural biology techniques, such as cryogenic electron microscopy (cryo-EM), are unraveling these mechanisms at unprecedented detail, providing a clear visualization of the receptors with the ligands that bind to them. This structural information can be used to directly inform the design of new analgesics with enhanced properties.
Structure-based design of opioid receptor agonists
While there are several types of opioid receptors, mu receptors (μOR) are most directly associated with both pain relief as well as dependence and addiction. μOR can act through two distinct signaling pathways, which utilize either G protein or β‐arrestin, respectively. Biasing the receptor toward the G protein pathway, through the use of specific ligands (agonists), has been shown to reduce unwanted side effects as well as lower addiction potential.
A study led by researchers at Stanford University and the University of Erlangen-Nuremberg investigated the structural basis of this behavior. They determined the cryo-EM structure of μOR bound to PZM21, a computationally determined agonist for G protein biasing. Their results provided structural validation of this agonist’s behavior and a potential starting point for the development of improved analgesics.

By directly visualizing agonist binding, cryo-EM supports the development of functional selective ligands.
Rational design of bitopic ligands targeting the mu opioid receptor
Bitopic pharmaceuticals consist of two molecular structures that are recognized by the drug target, joined by a linker. By interacting with multiple sites on a receptor simultaneously, bitopic ligands can more effectively and narrowly modulate receptor behavior than would be possible by introducing the two molecules separately. A collaboration led by researchers at the University of Washington developed novel bitopic ligands that couple the opioid fentanyl with a positively-charged guanidino group.
Attached in this way, the ligand is able to bind to the opioid receptor while also attaching to an allosteric sodium binding pocket. This served to greatly reduce μOR recruitment of β-arrestin while also increasing its preference for G protein binding. The design of this ligand, and its interaction with the allosteric binding site, was structurally validated with cryo-EM.

With cryo-EM, researchers are able to directly visualize the binding of bitopic ligands to their targets. Here, two bitopic ligands, with varying linker lengths, are shown in the binding pocket of μOR.
More recent studies have sought to further refine the behavior of these bitopic ligands, particularly the C6guano ligand shown above. A recent publication in ACS Central Science examined a novel ligand called RO76, where the guanidino group is replaced with a benzyl alcohol. Researchers found that this ligand showed similarly reduced side effects as C6guano while also having increased ability to pass through the blood-brain barrier. Once again, the structural interaction of ligand and receptor was visualized with cryo-EM analysis.
Drug design targeting the kappa opioid receptor
Most analgesic opioids target μOR, but there are several additional types of opioid receptor that are being investigated as potential alternate analgesic targets. Among them is the kappa opioid receptor (KOR), which is notable for its reduced addiction potential. Despite this, there are still challenges, as KOR binding is associated with a number of serious side effects. Drugs that target KOR must therefore have high specificity while also finding ways to mitigate these effects. Researchers applied a computation de novo approach to find prospective candidates consisting of peptide-small molecule conjugates. This approach was able to screen a large quantity of potential conjugates for best candidates, greatly reducing the typical lead optimization and screening required in drug discovery.
The resulting compound was structurally validated using cryo-EM, as the simulated interaction between the drug and receptor could be compared to cryo-EM visualization. In vivo efficacy studies were also performed to verify the behavior of the drug. Overall, this approach provides an exciting method for the discovery of highly selective ligands that target KOR, and could potentially be applied to any number of drug discovery challenges.
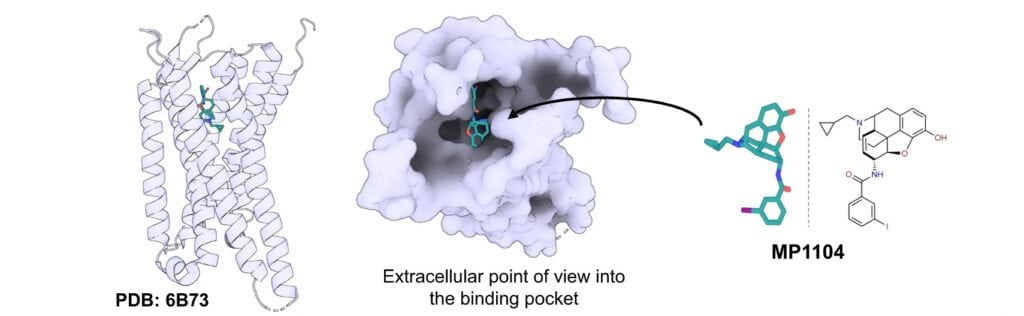
Peptide-small molecule conjugates investigated by Muratspahić et al. Figure reproduced under CC BY 4.0.
Managing pain and combatting opioid misuse through structure-based drug design
The opioid crisis is a complex and terrible consequence of the misuse of medicines that are intended to relieve pain and suffering. Addressing this crisis will require a multifaceted approach that has novel therapeutics at its core, because people will ultimately still require tools for pain management. With structure-based drug design, researchers are able to modulate these substances at a molecular level, greatly increasing the control that they can exert on a drug’s simultaneous side effects. Cryo-electron microscopy accelerates this rational design approach with high-resolution visualization of molecular interactions. We hope that novel lead compounds discovered with cryo-EM continue to pave the way to a future where medicines for pain relief provide only that, relief.
Learn more about cryo-EM in rational drug design >>
Marc Casper discusses how Thermo Fisher Scientific is helping address the opioid crisis >>
Alex Ilitchev, PhD, is a Science Writer for Thermo Fisher Scientific.
Written in collaboration with Dominic Meusch, Director of Segment Strategy, Thermo Fisher Scientific and Mark Barret, Senior Market Development Manager, Thermo Fisher Scientific.

Leave a Reply