Despite falling mortality rates, malaria continues to severely and disproportionately devastate the people of Africa and South Asia, causing nearly half a million annual deaths worldwide, with over nine in ten cases occurring in Sub-Saharan Africa. Worse still, according to the World Health Organization, the disease predominantly affects children under five, with roughly 70 percent of deaths occurring in that age range. While preventative measures such as treated mosquito nets have been critical for reducing incidents of the disease, the discovery of reliable, targeted treatments is still of paramount importance.
What we know about malaria
While infamously transmitted by mosquitos, malaria is actually caused by single-celled parasites from the Plasmodium genus. These bind to blood cells and fundamentally change their cellular behavior, supporting replication of the parasite in bubble-like compartments called “parasitophorous vacuoles” (PV). Both the interaction of the parasite with the blood cell and the growth of the PV are extremely promising drug targets, but a complete understanding of these mechanisms at a molecular level is needed for informed drug design.
How EM can help
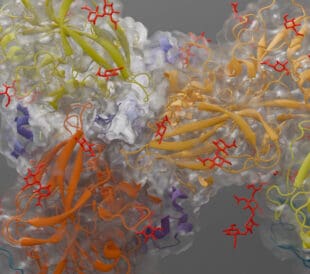
A recent collaborative project, spearheaded by scientists at UCLA, captured the single point of entry into the parasite PV, a protein complex called the Plasmodium translocon of exported proteins (PTEX). By analyzing thousands of isolated copies of the PTEX using cryo-electron microscopy single particle analysis (cryo-EM SPA), two principal forms of the complex were identified. Shifting between them creates the “feeding mechanism” by which PTEX passes parasite protein into the interior of the blood cell. Cryo-EM SPA not only imaged the entire structure of the complex but also identified the discreet location of its components; the EXP2, PTEX150, and HSP101 protein. EXP2 was determined to be the membrane spanning protein which facilitates parasite protein excretion, and its interaction with HSP101 is critical for PTEX function. Now that it has been isolated, this interaction can be specifically targeted by future drug design to deactivate the complex.

PTEX complex in engaged and resetting state, which facilitates cargo transport. adapted from Chi-Min Ho et al. Nature (561) 2018, PDB ID 6e11 and 6e10, visualized by Chimera
While cryo-EM SPA provides excellent resolution of large protein structures, imaging the entire PV as it grows requires observation of fine details at a cellular level. A group from the University of Melbourne has done just that by using serial block face-scanning electron microscopy (SBF-SEM), an EM technique that images and then takes sequential slices off the surface of a sample. These stacked images are combined to form a 3D representation. This method allowed Molly Parkyn Schneider and her team to visualize the various stages of PV development and maturation. In particular, they were interested in the process by which the parasite prepares for replication. In its final stage, the PV adopts a characteristic crescent or banana-like shape, which is primed for gamete formation. Protein knockout studies combined with SBF-SEM revealed that a pair of protein, PhIL and PIP1, are jointly necessary for growth of the microtubule complex by which the PV expands. By targeting these proteins the replication of PV could be halted, stopping the disease in its tracks.

3D reconstruction of PVs at various stages of maturity. Adapted from Molly Parkyn Schneider et al. PLOS Pathogens 13(10), October 6, 2017.
Combined, these two EM methods have provided an incredible picture of disease progression, from initial interaction between parasite and cell to its gradual growth therein. These novel insights have identified specific targets for future drug design, hopefully leading to faster drug development and availability.
Subscribe now to receive new Accelerating Microscopy posts straight to your inbox. For more information about Thermo Scientific microscopy instruments, visit thermofisher.com/em.
Gabriella Kiss, PhD, is a Product Marketing Manager for Single Particle Workflow at Thermo Fisher Scientific.
To learn more about cryo-EM or microscopy, fill out this form to speak with an expert.


Leave a Reply