Cytogenetics has been a key part of biology since 1842, when Swiss botanist Karl Nägeli first discovered chromosomes in pollen. In the decades since, the science has been defined as the study of chromosomes, including their behavior, mechanics, and role in inheritance. Ever since Nägeli’s discovery, methods for examining chromosomes have become more and more effective, further illuminating their roles in cell biology and human and animal health in ways undreamed-of when chromosomes were first discovered.
In 1870, German anatomist Walther Flemming introduced aniline staining to observe chromosomes during cell division for the first time. Aniline is a poisonous substance derived from coal tar and, nowadays, is mostly used as a precursor for the synthesis of other organic compounds. For Dr. Flemming, it provided a way to preferentially mark nucleic acids and make them accessible to microscopy, and his illustrations provide some of the first images of chromosomes. Indeed, the propensity of chromosomes to accept dyes like aniline is why they are called chromosomes, or “color bodies.” Aniline was enough to observe the stages of cell division and provide a clear indication that chromosomes have a special role in it, but it could not offer much more information. With whole chromosomes colored essentially the same way and with staining not working on still-living cells, other methods were necessary.
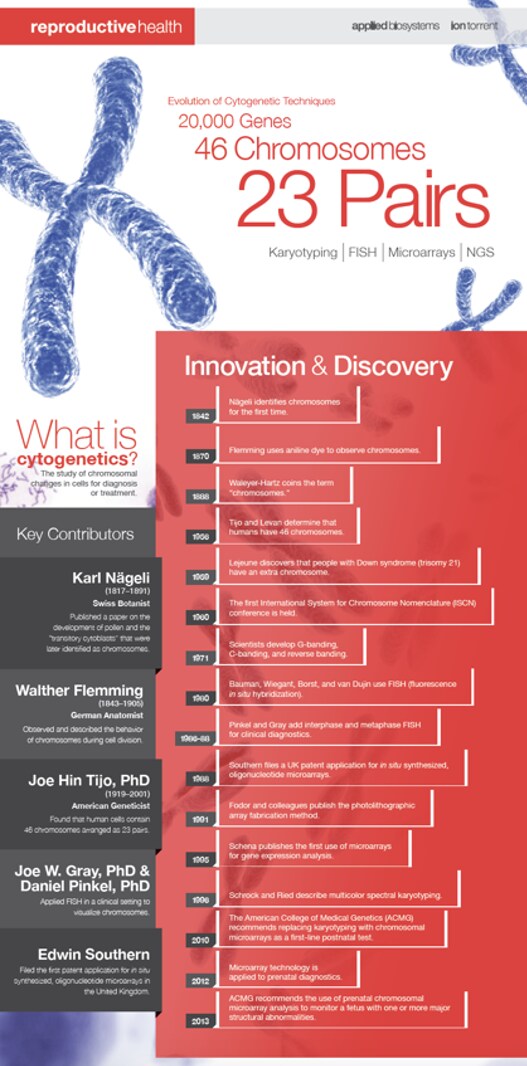 Efforts to ascertain the number of chromosomes in human cells took decades in the early to mid-20th century. In 1923, Theophilus Painter1 derived an estimate of 48 chromosomes using images of nuclei reconstructed from thin sections of human testicular tissue embedded in paraffin and stained with iron hematoxylin, and this estimate stood until 1956. In that year, Tjio and Levan2 used improved methods to show that human cells have 23 pairs of chromosomes, overturning Painter’s estimate. Tjio and Levan’s methodological improvements included using squashed rather than sectioned tissue, freezing cells in metaphase with colchicine, and using hypotonic solutions to make individual chromosomes easier to resolve. Their achievement would later be confirmed with other karyotyping methods.
Efforts to ascertain the number of chromosomes in human cells took decades in the early to mid-20th century. In 1923, Theophilus Painter1 derived an estimate of 48 chromosomes using images of nuclei reconstructed from thin sections of human testicular tissue embedded in paraffin and stained with iron hematoxylin, and this estimate stood until 1956. In that year, Tjio and Levan2 used improved methods to show that human cells have 23 pairs of chromosomes, overturning Painter’s estimate. Tjio and Levan’s methodological improvements included using squashed rather than sectioned tissue, freezing cells in metaphase with colchicine, and using hypotonic solutions to make individual chromosomes easier to resolve. Their achievement would later be confirmed with other karyotyping methods.
Starting in the 1970s, fluorescent staining provided much more information. Researchers developed three techniques for staining chromosomes in a way that provided structural information, revealing them to be complex structures. C-banding stains highly repetitive cytosine-rich regions, most often the centromeres where the chromosomal arms meet, revealing these regions. G-banding, similarly, uses Giesma stain to mark heterochromatic regions, which are tightly wound and less transcriptionally active. Q-banding uses quinacrine for similar results, but also fluoresces in proportion to the AT enrichment of the part of the chromosome to which it binds, providing more information. These staining methods provided critical insight into the complexity of the information stored in chromosomes and hinted at how much more could be learned about them.
G- and q-banding enabled the critical innovation of karyotyping. Once chromosomes could be clearly distinguished from one another, they could be sorted and compared. This practice, once performed with photographs and scissors, confirmed Tjio and Levan’s chromosome count and indicated that one pair, deemed X and Y, is related to sex determination. These insights helped elucidate the different roles of various chromosomes, by enabling researchers to observe that damaged or missing chromosomes led to specific genetic conditions, most famously Down syndrome from a third copy of chromosome 21.
The next key innovation was fluorescence in situ hybridization (FISH), developed in the early 1980s. Similar in concept to the banding techniques that preceded it, FISH uses fluorescent molecules attached to DNA or RNA probes. These probes bind to their complementary sequence on chromosomes, and their fluorescence can then be observed. Depending on the probes selected, FISH can therefore reveal the number of chromosomes, the presence or absence of particular sequences, translocation events in which a particular chromosome region has moved to an unusual location, and more. More recently, multicolor-FISH (mFISH) allows multiple probes with different fluorescent markers to be used in the same analysis, allowing more complex data collection. FISH continues to be useful for many applications, including species-level identification of seafood for enforcement purposes.
Fluorescence in situ hybridization contributed to one other breakthrough in cytogenetics in the early 1990s: comparative genomic hybridization (CGH). In this variation, multiple labeled DNA probes are allowed to hybridize with the sample of interest, and the hybridization of each one is directly compared to the others. Typically, the probes have different labels and correspond to different versions of the same stretch of target DNA, such as different alleles or disease states. Thus, the comparison between them reveals whether the sample’s chromosomes preferentially hybridize with one or the other. Combined with karyotyping, this can show whether target DNA is located in an unusual place in a sample, and with a confirmed normal control sample, it can detect copy number variations, which are a critical part of numerous genetic conditions.
The current cutting edge of cytogenetics is microarrays. Microarrays are, in essence, microscope slides with thousands of tiny spots on which DNA or RNA probes are attached. The probes can be fluorescent or otherwise similar to the probes used in other techniques. By concentrating probes in this way, a single sample can be checked for thousands of different targets, and this technique can be adapted for many different kinds of probes, including single nucleotide polymorphisms (SNPs), copy number variations (CNVs), and more. The use of discrete wells means that microarray data can be easily digitized, making for particularly rapid and thorough data collection.
Microarrays may seem like they completely replace all of their predecessors, but the future of cytogenetics isn’t in a single technique. Most of the methods developed in the late 20th century are still relevant in the 21st, whether in limited contexts or to augment the information provided by more modern techniques. Techniques developed for genomics, such as next-generation sequencing (NGS), also provide insight into the same sorts of questions that cytogenetics pursues. Future innovations in cytogenetics will combine what works about all of the past’s techniques to find newer, more efficient ways to collect cytogenetic information, as well as new tricks for answering questions that still vex modern practitioners.
For more information on prenatal and postnatal genetic testing, visit our education page here
Resources:
1. Painter, T. S. (1923). Studies in mammalian spermatogenesis. II. The spermatogenesis of man. J. Exp. Zool. 37, 291–336.
2. Tjio, J. H. & Levan, A. (1956). The Chromosome Number of Man. Hereditas 42, 1–6.
Leave a Reply