In our new four-part blog series, we explore why you should implement a laboratory information management system (LIMS) in your lab, what factors you need to consider in the selection process, and how to plan your implementation. Our last blog highlighted the key capabilities that a LIMS offers — here we explore how to pick the right LIMS.
A LIMS can open up a plethora of opportunities for your lab. Whether you need to ensure product quality, demonstrate regulatory compliance, manage raw materials, or all three, a LIMS has the capabilities to help. But each lab has different requirements — which can also change over time — so you should choose a LIMS that brings the most benefit to your lab now and in the future.
LIMS are generally flexible and designed to support the requirements of large and small labs alike, from academic start-ups to enterprise-level development. But selecting the wrong LIMS can actually hinder your lab processes, as you spend time and resources struggling to shoehorn your workflows into a system not designed for it.
So, which LIMS should your lab use? One critical factor to consider is whether you need to manage data for R&D or Process Development and Manufacturing. Here we explore our two different LIMS offerings: Thermo Scientific™ Core LIMS Software, and Thermo Scientific SampleManager™ LIMS, lab execution system (LES), electronic notebook (ELN) and scientific data management system (SDMS) software, and examine how they help different industries such as pharmaceuticals and mining.
Define, capture and manage R&D lab data
In some labs, R&D is at the core of every single process. Here, it is important to select a LIMS that is designed for managing R&D-related lab data and workflows.
Thermo Scientific Core LIMS Software does just that, for any unregulated laboratory. Our Core LIMS software collects, shares, analyzes, and archives scientific data relating to your R&D processes. But it is also flexible and easy to adapt. As science and technology advances and your lab adopts new instruments, techniques and data types in response, our Core LIMS software evolves too.
A LIMS for pharma and biopharma labs
(Bio)pharmaceutical R&D labs must be agile, as they need to manage diverse data types in high volume. Core LIMS software supports these labs with all their workflows, including:
- Sample and inventory management, notifying the labs when consumables are running low.
- Sample preparation and analysis, keeping track of all key data.
- Instrument and software integration, helping move samples through different workflows.
- Workload management, maximizing productivity and helping work around instrument downtime.
- Reporting and data analytics, capturing all relationships and metadata for working with data in context.
- Dashboards and data visualization, providing configurable insights into your data at-a-glance.
Ensure quality and compliance in Process Development and Manufacturing
Process Development and Manufacturing labs need a comprehensive solution that supports lab data and process management across a range of functions with minimal effort. Thermo Scientific SampleManager LIMS, LES, ELN and SDMS software allows labs to quickly build workflows that map to actual lab processes, while also automating decisions and actions to reduce the need for user intervention.
Additionally, the software facilitates the strict compliance to various standards (such as 21 CFR Part 11, GMP and ISO 17025) required of Process Development and Manufacturing labs. Figure 1 demonstrates how the different software connects the entire process together.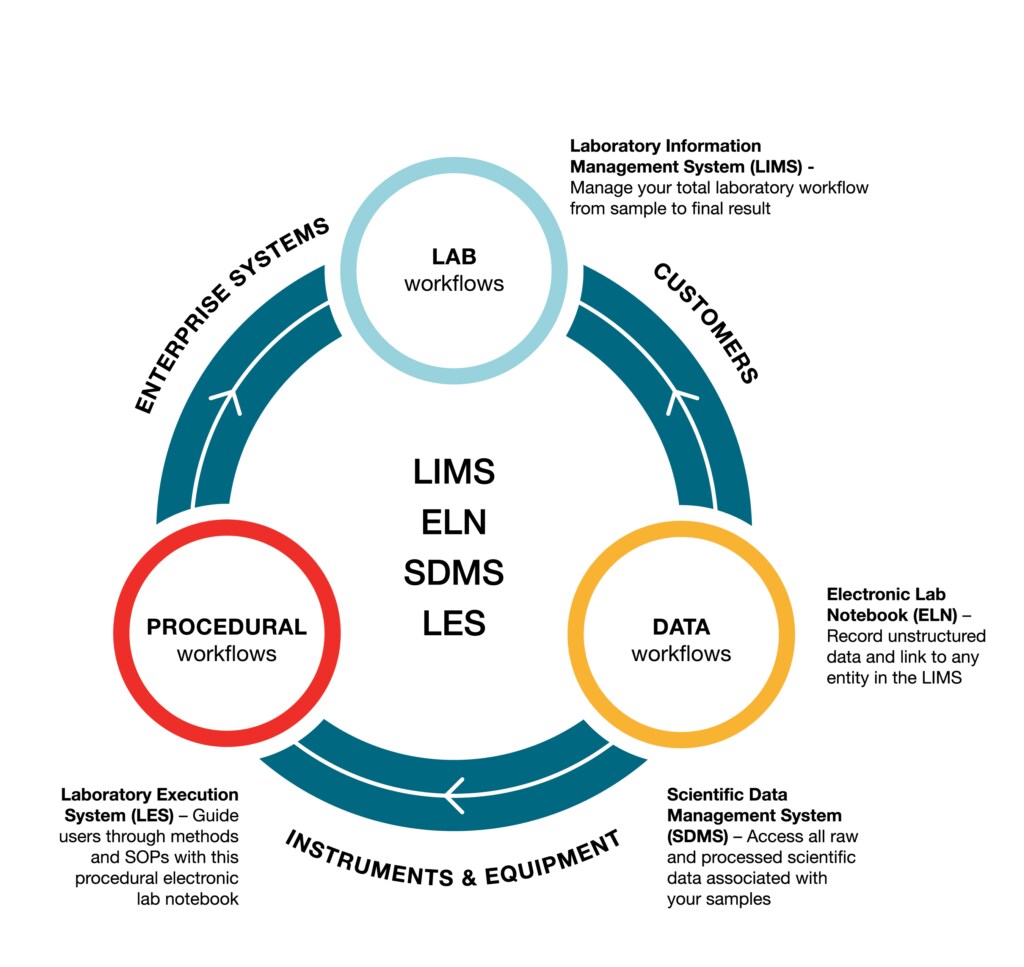
Figure 1: Schematic to show how SampleManager LIMS, LES, ELN and SDMS software fits together to support Process Development and Manufacturing.
A LIMS for manufacturing QA/QC
Manufacturing companies need to verify their end-product quality, and also adhere to local environmental and government regulations. SampleManager LIMS, LES, ELN and SDMS software can support these organizations to:
- Improve efficiency and quality from raw materials to final processing.
- Completely automate their workflows by connecting with lab instruments and enabling their control.
- Deliver scalability and reliability by connecting the lab to the production facility.
- Track, measure and report critical events and actions in real time.
- Comply with industry regulations by producing full audit trails.
Experience greater lab efficiency with LIMS
Whether you’re working to discover or create, a LIMS can improve the efficiency of your lab, reduce hands-on time and streamline your workflows. But not every lab is the same, and the LIMS that is most appropriate for one lab may not meet the needs of another. Determining which solution can address all the requirements of your lab is the key to unlocking greater productivity, efficiency and ease.
Now you’re aware of a key consideration when choosing a LIMS, keep an eye out for the final blog in our series where we explore how to ensure smooth and efficient implementation. To discover more about selecting the right LIMS, download our eBook today.
To whom it may concern,
We are currently in the later stages of the process of hopefully attaining ISO17025 accreditation for testing soils for pH, lime requirement, phosphorous and potassium in our onsite laboratory. We are now looking to install a LIMS system. Would it possible to speak to someone on this and book a demo and have someone come to our site?