
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) is the most significant expansion of the FDA’s authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic (FD&C) Act was passed in 1938. This new law in the United States of America will help ensure the safety of cosmetic products many consumers use daily.[1]
MoCRA introduces several key provisions that impact cosmetic manufacturers and businesses. One of the primary goals of the legislation is to enhance the FDA’s oversight and regulatory framework for cosmetics. It empowers the FDA to conduct safety assessments of cosmetic ingredients, establish good manufacturing practices (GMPs), and require pre-market approval for certain high-risk products.
Cosmetic manufacturers are required to provide, support, and manage:
- Ingredient lists and accurate product labels
- Safety data
- Information on the manufacturing process
- Recalls of unsafe products
To remain compliant with the MoCRA legislation, cosmetic manufacturers can utilize Thermo Scientific™ SampleManager™ LIMS Software to assist with regulatory compliance. SampleManager LIMS will streamline the process of gathering and organizing the required information, ensuring that manufacturers have the necessary documentation readily available for FDA inspections and audits.
GMP support
Good manufacturing practice (GMP) compliance is crucial under MoCRA, as it ensures the quality and safety of cosmetic products. SampleManager LIMS can help manufacturers implement GMP practices, such as raw materials and finished product lot management, batch tracking, quality control, and documentation of manufacturing processes. This can assist in reducing errors and ensuring consistency in production, ultimately aiding in compliance with MoCRA’s GMP requirements.
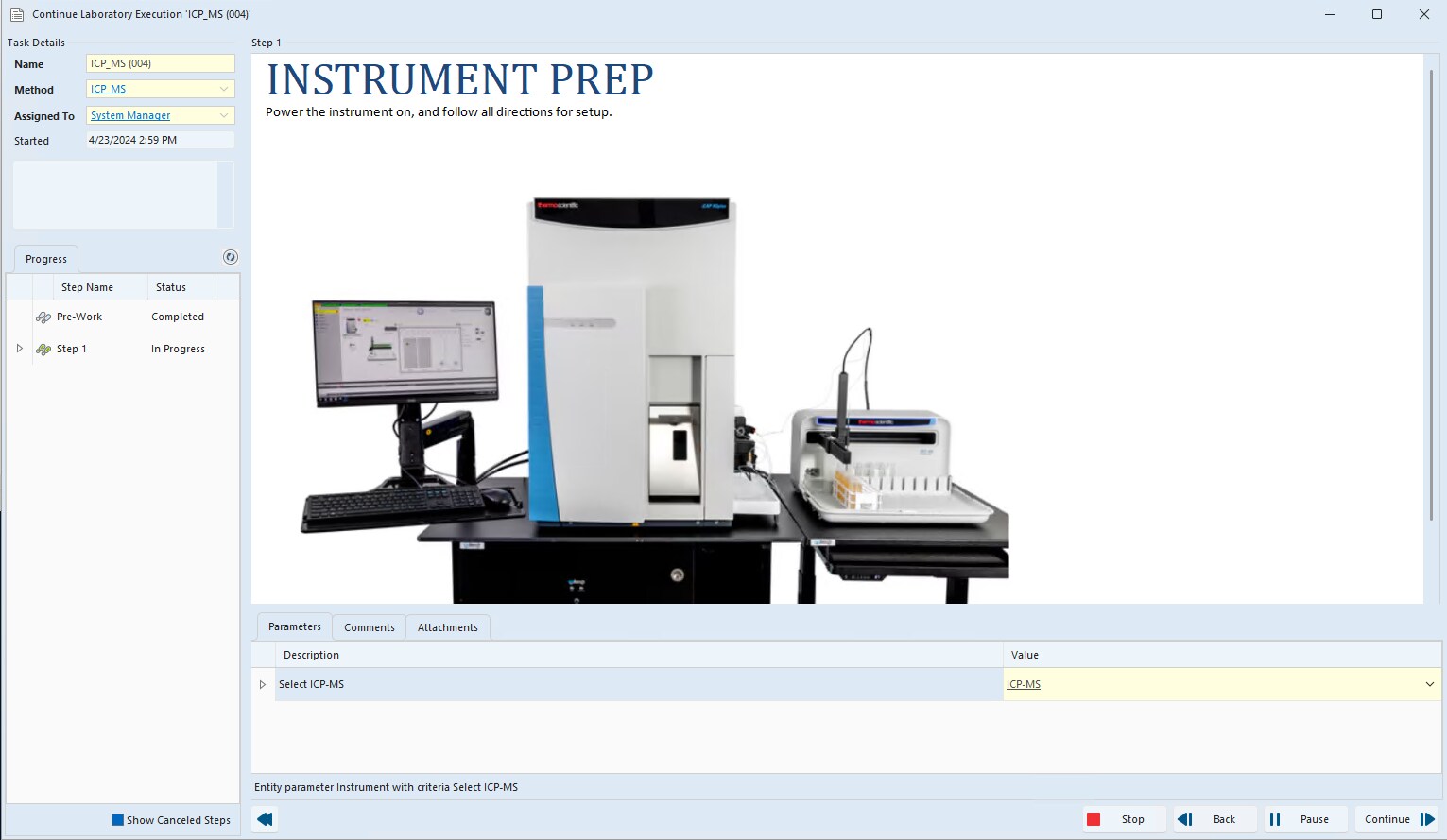
Figure 1: The Lab Execution System (LES) in SampleManager software supports consistent traceable, repeatable testing.
Product labeling
SampleManager LIMS can provide tools for managing product labeling and packaging compliance. MoCRA introduces stricter requirements for labeling, including the provision of specific information such as product function, directions for use, and warnings. By utilizing SampleManager LIMS, manufacturers can create and maintain compliant labels, ensuring that all necessary information is included and displayed correctly.
Traceability for adverse events and recalls
SampleManager LIMS can also aid in maintaining records of adverse events and conducting recalls. MoCRA requires manufacturers to report any adverse events associated with their cosmetic products to the FDA and perform recalls. SampleManager LIMS can streamline this reporting and recall process, allowing manufacturers to efficiently identify lots and batches of materials affected by adverse events.

Figure 2: Raw materials are traceable all the way through to finished product.
Environmental monitoring
Finally, SampleManager LIMS can help streamline environmental monitoring programs. By automating sample point schedules and managing the process of both testing and sample send outs to external laboratories, the Environmental Monitoring Solution for SampleManager LIMS will reduce the time and effort required to manage the environmental monitoring process in the manufacturing facility.

Figure 3: Sampling points are easily managed, and issues highlighted for Environmental Monitoring programs.
Streamline your processes
In summary, the MoCRA legislation represents a significant update to the regulatory framework for cosmetics in the United States. By utilizing SampleManager LIMS Software, a QA/QC LIMS tailored to regulatory compliance, cosmetic manufacturers can streamline their processes and ensure adherence to the new requirements. From managing raw materials and finished product lots to environmental monitoring program to implementing GMP practices and maintaining compliant labeling, SampleManager LIMS can be a valuable tool in helping manufacturers remain compliant with the MoCRA legislation.
 Figure 4: SampleManager LIMS supports the complete cosmetics production workflow.
Figure 4: SampleManager LIMS supports the complete cosmetics production workflow.
Let SampleManager LIMS help you save time, reduce errors, achieve optimal productivity, and revolutionize your laboratory operations. To learn more, please visit our website. Alternatively, if you would like to discuss how SampleManager LIMS can support your lab today, please feel free to contact us.
[1] Modernization of Cosmetics Regulation Act of 2022 (MoCRA) | FDA
Leave a Reply