 Did you know that clove tea has been used to relieve nausea, and that clove oil can be used to reduce the presence of Listeria monocytogenes and Lactobacillus sakei in food? According to the University of Hawai’i:
Did you know that clove tea has been used to relieve nausea, and that clove oil can be used to reduce the presence of Listeria monocytogenes and Lactobacillus sakei in food? According to the University of Hawai’i:
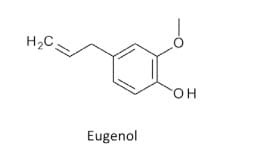
Clove buds yield approximately 15% to 20% of a volatile oil that is responsible for the characteristic smell and flavor. The bud also contains a tannin complex, a gum and resin, and a number of glucosides of sterols. The principal constituent of distilled clove bud oil (60% to 90%) is eugenol (4-allyl-2-methoxyphenol). The oil also contains about 10% acetyleugenol and small quantities of gallic acid, sesquiterpenes, furfural, vanillin, and methyl-n-amyl ketone. Other constituents include flavonoids, carbohydrates, lipids, oleanolic acid, rhamnetin, and vitamins….
Clove oil is reported to have antihistaminic and antispasmotic properties, most likely due to the presence of eugenyl acetate. Cloves are also said to have a positive effect on healing stomach ulcers…. As with many other volatile oils, clove oil inhibits gram-positive and gram-negative bacteria.
Eugenol, the main ingredient in clove oil, is a familiar fragrance in many dental offices as it is often mixed into a paste and used in dentistry as a local antiseptic and anesthetic. It is a pale yellow oil with a warm, pungent, yet pleasing aroma, the smell of bay leaves and clove. Eugenol concentration in clove oil is as high as 90%. Eugenol is also found in bay leaves and allspice, and other botanical oil, but in lower concentrations.
Essential oils are extracted, depending on the nature of the botanical material, by a variety of techniques, including expression (cold-press), solvent, enfleurage (cold-fat) and supercritical fluid (SCF) extraction, and distillation. Most essential oils are extracted by direct or indirect steam distillation.
Steam distillation is a co-distillation technique that uses live steam to separate components of a mixture. It is effective at extracting high-boiling-point components of essential oils, where boiling points are as high as 200°C. Yet the oil vapors themselves are closer to 100°C, thus helping to preserve the structural integrity of the compounds. It allows distillation to be performed at temperatures below the boiling points of the individual components.
Indirect steam distillation is a technique used for generating steam in situ, where the water level is kept below the plant material. Co-distillation is the distillation of components of a mixture that are immiscible with water. Steam vaporizes the high-boiling essential oils and the hot vapors condense back into a liquid, along with water, as they pass through a cooling system. Since the oils are immiscible in water a two-phase distillate is produced, a water layer and an oil layer. The oil is usually less dense and floats atop the water. The aqueous layer can then be siphoned off using a separatory funnel. If an emulsion develops, as is often the case in small-scale distillation, the immiscibility of the hydrophobic oil makes extraction and isolation of the product with a non-polar organic solvent highly effective.
 We have developed a lesson plan for chemistry teachers to conduct an experiment involving the extraction of eugenol from whole cloves by co-distillation with indirect steam, steam that is generated in situ. During the process, Eugenol is extracted from the distillate with dichloromethane and analyzed using NMR spectrometers.
We have developed a lesson plan for chemistry teachers to conduct an experiment involving the extraction of eugenol from whole cloves by co-distillation with indirect steam, steam that is generated in situ. During the process, Eugenol is extracted from the distillate with dichloromethane and analyzed using NMR spectrometers.
NMR spectroscopy is the method of choice for many organic chemists because of its versatility in elucidating molecular structure, optimizing reaction dynamics, measuring reaction kinetics, monitoring reaction content and controlling product purity. NMR spectroscopy provides a wealth of information on physical, electronic, chemical and structural aspects of molecular systems. It is a nondestructive technique ideally suited for measuring and recovering small sample quantities.
The challenge for the students involved in the clove experiment is to extract sufficient quantities of eugenol. Collecting at least 60 mL of distillate can yield up to 2 mL of eugenol after extraction and evaporating off dichloromethane. The experiment, which is moderately difficult, takes about five hours to do the procedures and analysis. As with many experiments, safety precautions should be taken. Eye protection should be worn at all times during the experiment and each wall outlet used must be equipped with a 3-pronged grounded outlet, with the ground being a noncurrent-carrying wire connected to earth ground at the main distribution box.
The instrument procedure for sample analysis using an NMR spectrometer includes: shim, prepare, inject, acquire, analyze. You can access and download the lesson plan here: Extraction of Eugenol from Cloves lesson plan.
Explore our online Chemistry in the Classrooms to find additional lesson plans, videos and products that help empower students and teachers through instrumentation and resources.






Leave a Reply