What’s next for electroporation? Discoveries to date suggest we’re in for an exciting ride.
By Lisa Kepler
Cell membranes are tricky little things. Membrane permeability is an essential feature for novel therapeutic candidates, but getting genetic material and other molecules into cells is a little like trying to add water to a sealed water balloon. Spray a hose and the water will just bounce off the surface; pierce the balloon and you risk losing the good water already there. Scientists have come up with a number of clever ways to cross the membrane barrier while keeping the cell intact, each with their own pros and cons. But a standout method for its high efficiency, broad applicability, and proven safety is electroporation.
Shepherding the next era of cell and gene therapy
Electroporation is a non-viral technique that involves using brief, high-intensity electrical pulses to create transient pores in the membrane of cells. When the electrical pulses are turned off, the membranes reseal so the cell can survive. It’s a phenomenon that has been observed since the 18th century, but today’s greater understanding and control of the parameters has made it a powerhouse for scientific discovery [1]. Now with excellent transfection efficiency, expanded applicability, and enhanced cell viability, electroporation has established itself as a promising method to fuel genetic engineering, gene therapy, cell reprogramming, and the development of novel therapies and treatments.
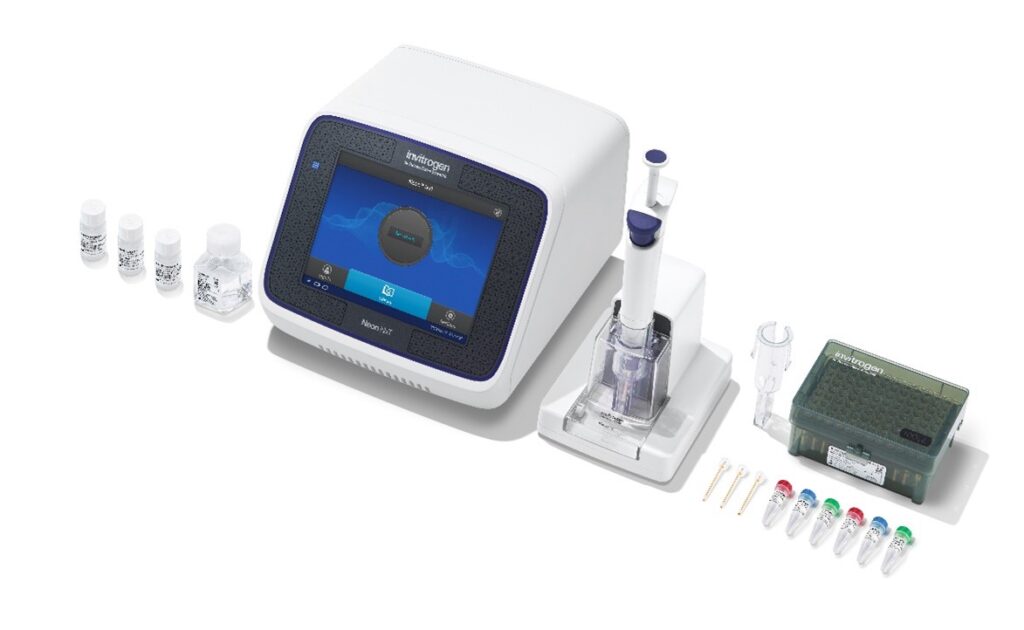
And with the introduction of the Invitrogen™ Neon™ NxT Electroporation System, it’s about to get even better.
Neon NxT is a next-generation electroporation platform that builds on the same core technology as the legacy Neon Transfection System to help streamline the cell transfection workflow. Users who love Neon may be happy to know that Neon NxT continues to offer an impressive suite of benefits designed to help researchers up their electroporation game:
- Optimized cell viability: Optimized electrode design and fewer pipetting steps in Neon NxT help increase transfection efficiency and improve cell viability.
- Exceptional sample protection: Neon NxT’s compact design and unique pipette tip help minimize the risk of sample
- Improved productivity: Invitrogen TransfectionLab, an app found on Thermo Fisher Connect, brings cloud connectivity to Neon NxT and allows for remote set up of multiple plate layouts with direct access to any protocol saved in the app.
- Simplified workflow: Despite its sophisticated design, Neon NxT is surprisingly easy to use. A simple-to-follow workflow can shorten the transfection process to 10-15 minutes.
- Flexible and customizable: The ability to modify parameters from pulse voltage to payload can enable researchers to precisely control their experiments.
Neon NxT builds on the best of the legacy Neon system and is well positioned to help accelerate innovation. With up to 90 percent transfection and gene-editing efficiency in extremely difficult-to-transfect cells, it’s set to fill a critical gap in an industry ripe for disruption.
Five ways electroporation has already changed the world
So, what new discoveries will electroporation with Neon NxT empower? That remains to be seen, but a look through our protocol library at discoveries to date fueled by the technique makes one thing clear: the promise of electroporation knows no bounds.
Here are five ways electroporation has already propelled the field of cell and gene therapy.
1. Preventing off-target effects from CRISPR-Cas9
CRISPR-Cas9 is a popular choice for gene editing in clinical contexts, and it has long been thought to be reasonably specific with limited genetic alterations in the vicinity of the target site. But in 2018, researchers from the Wellcome Sanger Institute in Hinxton, UK used Neon technology to show that DNA breaks introduced by single-guide RNA/Cas9 frequently caused deletions extending over many kilobases in mouse cells. The researchers also found lesions distal to the cut site and crossover events, leading them to believe that there might be many more different genetic variations caused by Cas9 than we currently know about. Some of these variations could potentially cause pathogenic issues when the editing is done on many cells that are actively dividing. This discovery was eye-opening and brought the need for strategy development to attenuate this adverse effect to the fore of the field [2].
- Cell line and type: Mouse embryonic stem cells, RPE1
- Payload: DNA, Cas9 RNP
- Read the full publication
2. Developing Trim-Away as a method to remove proteins in cells without prior modifications
Protein depletion is helpful in trying to understand how a protein functions in a biological system, but many techniques to target and break down proteins directly require modifying the protein or are limited in their application. To address these limitations, a research team at the Laboratory of Molecular Biology in Cambridge, UK, used Neon technology to develop a method called Trim-Away. Trim-Away uses a protein called TRIM21, which binds to antibodies attached to pathogens or abnormal proteins and triggers their degradation—but electroporation was critical to prove the technique worked. In the very first Trim-Away experiments, researchers used electroporation and microinjection to introduce an antibody and prove that it binds to the target protein. The technique was a success, allowing researchers to quickly and effectively remove specific proteins in different types of cells without the need for prior modifications.
Trim-Away has been successfully used to deplete various proteins in a short amount of time. For example, it was used to remove Rec8 in mouse oocytes, which previously required complex genetic techniques. It has also been used to deplete the signaling molecule NLRP3 in human macrophages, which was challenging with other methods [3].
- Cell line and type: NIH323, HEK293
- Payload: Antibody
- Read the full publication
3. Enabling efficient genome editing of CD4+ T cells
The ability to edit CD4+ T cells holds great promise for improving immune responses, developing targeted cancer therapies, and addressing genetic diseases related to these cells—but editing T cells is no easy feat. Up until 2015, many methods were just moderately effective, only causing small changes in the targeted genes. To improve the process, a research team at University of California, San Francisco began exploring the idea of deleting genes that act as checkpoints in the immune system and using Neon-powered electroporation to deliver Cas9 ribonucleoproteins (RNPs) into the CD4+ T cells to edit their DNA. With this approach, they were able to introduce specific changes in the DNA by using a template molecule and homology-directed repair. This allowed them to make precise modifications in genes related to HIV resistance and immune checkpoint regulation.
The researchers found that this Cas9 RNP technology could edit the DNA of CD4+ T cells with up to 20 percent efficiency, suggesting it could be a promising tool for correcting disease-associated mutations in the future. Their study was foundational in establishing the use of Cas9 RNPs for editing the DNA of CD4+ T cells, both for experimental purposes and potentially for therapeutic applications [4].
- Cell line and type: Human CD4+ T cells
- Payload: DNA, Cas9 RNP
- Read the full publication
4. Reversing drug resistance in colon cancer
Colorectal cancer (CRC) patients often receive a drug called 5-Fluorouracil (5-FU), but resistance to this drug is a major problem and can lead to treatment failure. Scientists have discovered small molecules called microRNAs (miRNAs) that can help overcome this resistance by regulating specific processes in CRC cells. However, delivering miRNAs safely and effectively to the target cells was a challenge until researchers leveraged electroporation.
The team introduced purified exosomes from donor cells loaded with 5-FU and miR-21 inhibitor oligonucleotide (miR-21i) into a 5-FU-resistant colorectal cancer cell line using Neon electroporation technology. The engineered exosomes not only effectively reduced the levels of miR-21 (which is associated with drug resistance), but they also affected several cellular processes, including decreased tumor growth. Excitingly, the combination of miR-21i and 5-FU delivered through the engineered exosomes also increased the effectiveness of treatment in 5-FU-resistant colon cancer cells, a discovery with the potential to enhance the effectiveness of future cancer treatments [5].
- Cell line and type: Exosome, multiple
- Payload: miRNA
- Read the full publication
5. Simplifying the TALEN construction process
Transcription Activator-Like Effector Nucleases (TALENs) have been hailed for their ability to precisely modify the DNA of living organisms, but TALEN construction can be lengthy. Researchers from Hiroshima University in Japan made key modifications to the traditionally used Golden Gate TALEN Kit to shorten the construction process by using a simpler vector. This streamlined approach allowed them to complete the entire process, from starting the construction to testing it in living cells, in just one week. Using the Neon electroporation system, the researchers tested the utility of their process by delivering the new TALENS into female stem cells. Their methods were found to be quicker than traditional methods that rely on yeast-based evaluation and provide an easier and more efficient way to use TALENs in various organisms for genetic research and other potential applications [6].
- Cell line and type: iPS 201B7; dermal, human
- Payload: DNA
- Read the full publication
As more discoveries like these make headlines, electroporation’s star will only continue to rise, solidifying its place as a game-changer for advancing gene and cell therapy. The introduction of Neon NxT is well timed to help the scientific community harness this momentum, offering them a simple, fast way to electroporate. What new scientific mysteries will it help illuminate? As we edge tantalizingly closer to major breakthroughs in cancer treatments, genetic engineering, immunotherapy and more, excitement is palpable to find out.
Learn more at thermofisher.com/neonnxt »
Request a Neon NxT demo or quote. »
References
1. (n.d.). Chapter 1. In M. Girgis (Ed.), Trends in Computer Science, Engineering and Information Technology (pp. 1-7). Retrieved from https://link.springer.com/chapter/10.1007/978-3-642-05420-4_1
2. Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements.Nat Biotechnol 36, 765–771 (2018). https://doi.org/10.1038/nbt.4192
3. Clift, D., So, C., McEwan, W.A.et al. Acute and rapid degradation of endogenous proteins by Trim-Away. Nat Protoc 13, 2149–2175 (2018). https://doi.org/10.1038/s41596-018-0028-3
4. Kim, H., Um, E., & Cho, S. R. (2016). Jung, N. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proceedings of the National Academy of Sciences, 113(8), 2070-2075. https://doi.org/10.1073/pnas.1512503112
5. Liang, G., Zhu, Y., Ali, D.J.et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol 18, 10 (2020). https://doi.org/10.1186/s12951-019-0563-2
6. Sakuma T.,Hosoi S., Woltjen K., Suzuki K., Kashiwagi K., Wada H., Ochiai H., Miyamoto T., Kawai N., Sasakura Y. et al. (2013). Efficient TALEN construction and evaluation methods for human cell and animal applications. Genes Cells 18, 315–326. https://doi.org/10.1111/gtc.12037
##
For Research Use Only. Not for use in diagnostic procedures.
© 2023 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
Leave a Reply