
by Taryn Jackson, Ph.D.
Estimated reading time: 13 minutes
Table of Contents
What is a microplate reader?
A microplate reader or plate reader is a laboratory instrument used to detect biological, chemical, or physical events in samples contained in microplates (also called microwell plates). These devices are essential for high-throughput screening in research, diagnostics, and drug discovery.
Microplate readers analyze samples using various detection methods, including:
- absorbance, measuring how much light is absorbed by a sample at a specific wavelength.
- fluorescence, detecting emitted light from fluorescent molecules.
- luminescence, measuring light emitted by a chemical reaction.
- Time-Resolved Fluorescence (TRF), enhanced fluorescence detection for sensitive applications.
- Fluorescence Polarization (FP), used in binding studies.
- Fluorescence Resonance Energy Transfer (FRET), detecting energy transfer between two fluorescent molecules, often used in molecular interaction studies.
- AlphaScreen/AlphaLISA, used in biochemical assays.
Plate readers may offer one or more detection modes. There are two main types of plate readers:
- Single-mode readers (specialized for one detection method)
- Multimode readers (capable of multiple detection methods, making them versatile)
Modern microplate readers often integrate with automation systems for high-throughput screening and real-time data analysis, making them crucial for advanced research uses. They may also incorporate different optical mechanisms for analysis.
Plate readers are useful for many research applications in drug discovery (e.g., high-throughput screening of potential drugs), molecular biology (e.g., DNA, RNA, and protein quantification), food safety and environmental monitoring (e.g., detecting contaminants or toxins), and more.
What are the common detection modes of microplate readers?
1. Photometry: absorbance and turbidimetry
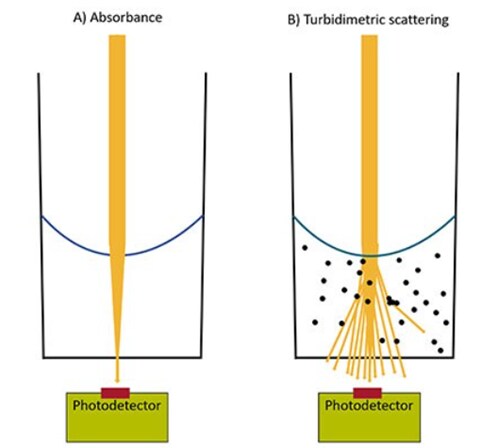
Photometric measurement using a microplate reader is based on the principle that photons of light can be either absorbed or scattered by a sample in solution. Measurement of light absorption is used in absorbance-based microplate assays, while measuring light scattering is used for turbidimetric microplate assays. Absorbance and turbidimetric measurements are both cost-effective and straightforward, suitable for a wide range of robust microplate assays.
Absorbance detection in microplate readers is used to measure the amount of light that is absorbed by a sample. When light passes through a sample, some of it is absorbed by the molecules in the sample, reducing the light intensity that reaches the detector. Since molecules absorb light a particular wavelength, they can be quantified based on how much light is absorbed. Visible absorbance assays utilize dyes that generate color in response to a reaction or molecular activity and can be monitored based on changes in the absorbance of the solution as the dyes become darker. UV absorbance assays do not require external dyes and are based on measurement of the intrinsic absorbance of a sample. Common applications for absorbance-based microplate reader assays include:
- Nucleic acid and protein quantitation at 260/280 nm
- Colorimetric protein quantitation assays (such as the BCA Protein Assays and Bradford Protein Assays)
- Enzyme-linked immunosorbent assays (ELISAs)
- Cell viability assays that generate a colorimetric signal in live cells (for instance, the CyQUANT XTT Cell Viability Assay)
Turbidimetric detection in microplate readers involves measuring the scattering of photons based on the presence of suspended particles or cloudiness of a solution. A higher concentration of particles in a sample will increase the turbidity and light scattering, which reduces the amount of light that reaches the detector when the sample is illuminated. Particles that cause light scatter can include cells, aggregates, precipitates, or other solids that are present in the solution. Bacterial growth curves are a common application that uses turbidimetric measurement.
2. Fluorometry: fluorescence intensity, FRET, and fluorescence polarization
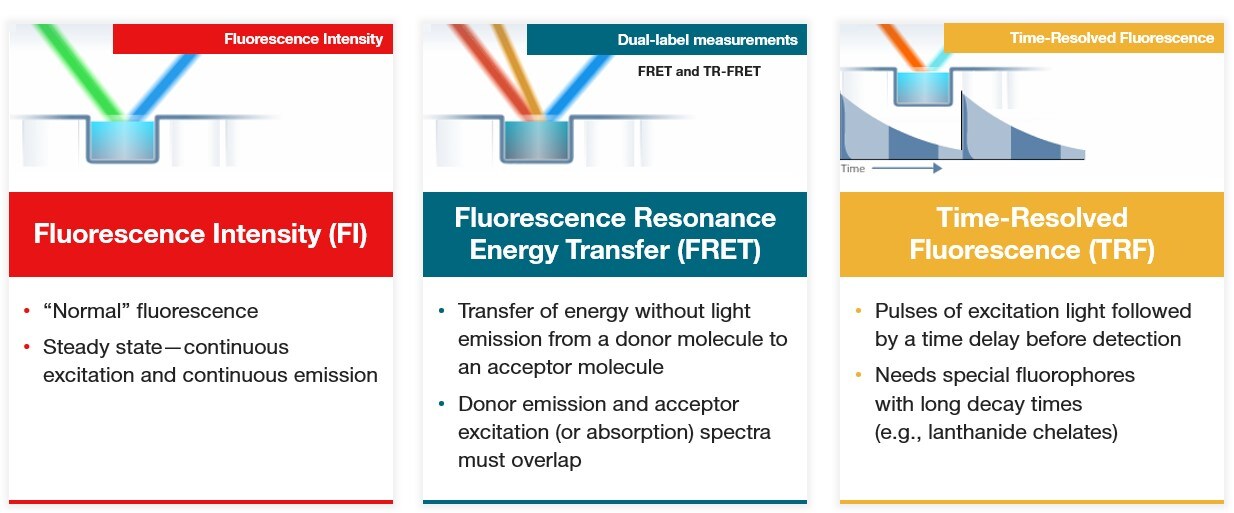
Microplate readers can be used for fluorescence detection by measuring the light emitted by fluorophores after they have been excited by a specific wavelength of light. Fluorophores, which absorb light at a certain wavelength and then emit light at a longer wavelength, can be attached to other molecules or used as indicators of a molecular activity or reaction. Fluorescent molecules have excitation and emission spectra, which enables their detection in microplate readers by illumination with light within their excitation spectrum followed by detection of the longer wavelength emission. Fluorescence intensity is linear to the fluorophore concentration and can be used for quantitative measurements. Fluorescence enables more sensitive measurements compared to absorbance and offers a wide dynamic range and ability to multiplex with different fluorophores. Common microplate reader applications that use fluorescence intensity include:
- Nucleic acid quantitation using fluorogenic dyes that bind DNA and RNA (Quant-iT DNA and RNA Assays)
- Cell viability and proliferation assays that use the reducing environment of living cells to convert resazurin to fluorescent resorufin (PrestoBlue and alamarBlue Cell Viability Reagents)
- Calcium flux assays
- Fluorescence reporter gene assays (GFP, YFP etc) for measuring gene expression and signaling pathway activation
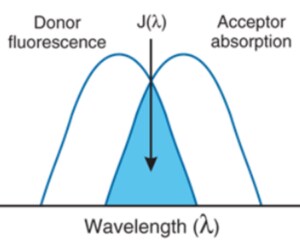
Multimode microplate readers are also used for specialized fluorescence techniques, such as Fluorescence Resonance Energy Transfer (FRET) and fluorescence polarization. FRET assays are based on two fluorescent molecules, a donor and an acceptor, that have energy transfer from one molecule to the other when they are in close proximity. The donor molecule absorbs excitation light and instead of emitting light, transfers it to the acceptor molecule, which then emits light at a longer wavelength. The donor emission and acceptor excitation must overlap for FRET to occur. FRET is commonly used for interaction studies to determine if two molecules bind to each other or are in close proximity. Fluorescence polarization is another fluorescence technique in which fluorophores are excited by polarized light and the polarization of light emitted is used to determine the orientation and mobility of molecules.
3. Time-resolved fluorometry: TRF and TR-FRET
Time-resolved fluorescence (TRF) is a specialized fluorescence technique that uses fluorophores with long fluorescence lifetimes, such as lanthanide chelates or cryptates. These molecules have fluorescence emission that is delayed and longer lasting compared to standard fluorophores, allowing excitation with short pulses of light followed by a time delay before emission detection. Because the emission detection is delayed from the excitation pulse, background fluorescence and autofluorescence from standard fluorophores is significantly reduced, increasing signal to noise.
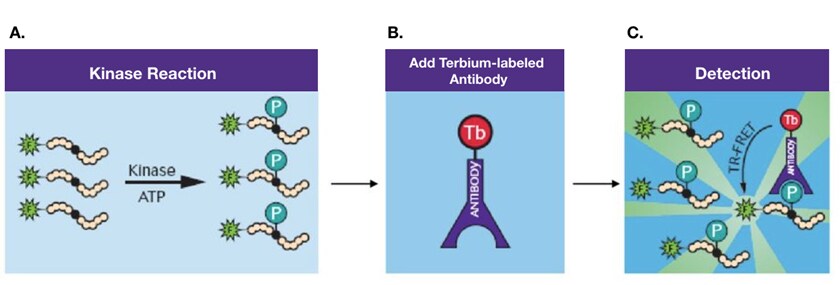
TRF is a very sensitive detection technology for microplate readers, often used in drug discovery microplate assays. Time-resolved Fluorescence Resonance Energy Transfer (TR-FRET) combines TRF and FRET to utilize energy transfer between a donor and acceptor fluorophore, which has delayed fluorescence emission upon excitation of the donor. TR-FRET is used in molecular interaction studies requiring high sensitivity and for detection of post-translational modifications such as LanthaScreen Kinase Activity Assays.
4. Luminometry: Luminescence and BRET
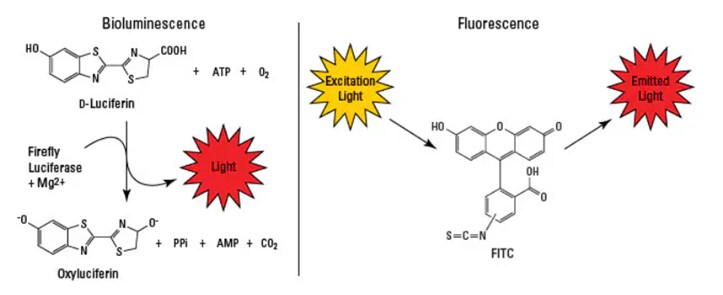
Microplate readers can be used for luminescence detection in which light is produced by a chemical or biochemical reaction, often involving a luminescent substrate and an enzyme. Compounds that are luminescent emit light at visible wavelengths and do not require an external light source for excitation. Luminescence can be classified as chemiluminescence, in which light is produced from a chemical reaction involving light-emitting compounds (such as luminol), or bioluminescence, which involves naturally occurring luminescence from biomolecules in light-emitting organisms (such as the light-producing enzyme luciferase found in fireflies).
Luminescence offers the highest sensitivity with low background for microplate assays. Flash luminescence assays produce a quick flash of intense light emission, while glow luminescence assays have a prolonged light emission that is more stable but lower intensity. Luminescence-based microplate assays are often used in high-throughput screening (HTS) applications due to their high sensitivity and simplicity. Common luminescence microplate reader applications include:
- Cell viability assays based on ATP measurement, which is required for luciferase to produce light (such as CellTiter-Glo and the ATP Determination Kit)
- Luminescence-based reporter gene assays for measuring gene expression and signaling pathway activation
Bioluminescence Resonance Energy Transfer (BRET) is a technique that is similar to FRET but involves the transfer of light emitted from a luminescent donor to a fluorescent acceptor.
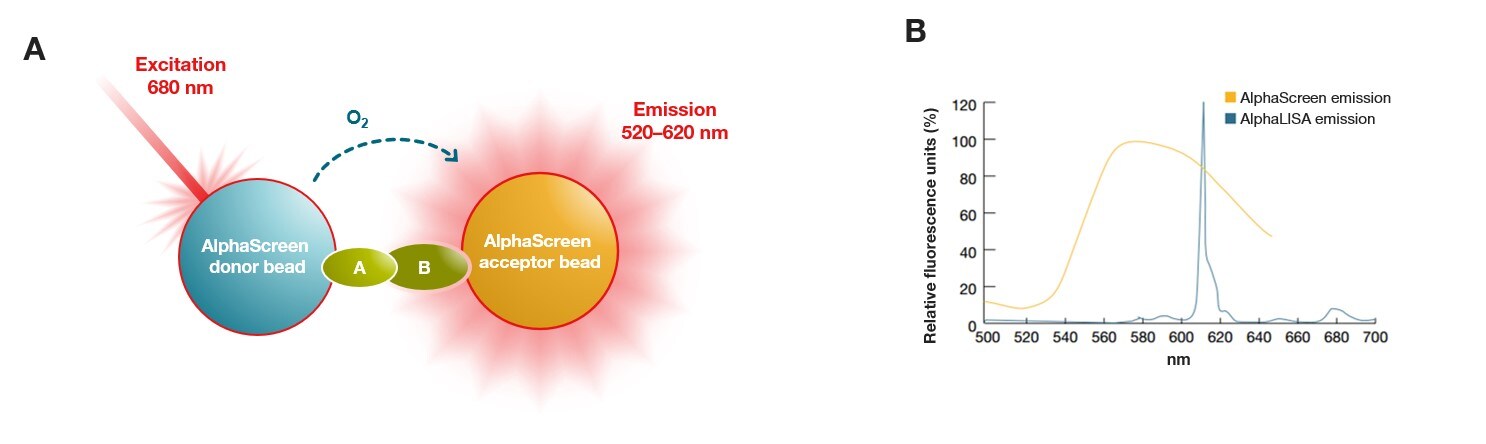
5. Alpha detection technology: AlphaScreen and AlphaLISA
Alpha detection technology is a bead-based binding assay for microplate readers that uses donor and acceptor beads to generate a luminescent signal upon proximity. Donor beads produce singlet oxygen when they are excited with red light at 680 nm. Singlet oxygen then diffuses to the acceptor beads, producing light emission at 520-620 nm that is detected by the plate reader. Molecules can be attached to the beads that bring the donor and acceptor beads close together upon binding, which initiates singlet oxygen transfer from donor to acceptor. Acceptor beads used in AlphaScreen microplate assays emit a broad spectrum of light from 520-620 nm, while AlphaLISA acceptor beads emit light with a narrow spectrum and brighter emission, enabling their use for microplate assays using samples that are complex and protein-rich such as serum or plasma.
In Alpha detection assays, the excitation wavelength is higher than the emission wavelength, eliminating background fluorescence and autofluorescence more effectively than in TRF. Alpha detection assays are also easier to set up and design compared to other proximity assays such as TR-FRET and BRET, which require careful optimization. Because Alpha detection assays are homogenous and do not require separation or washing of the bound and unbound molecules prior to detection, they are often used in high-throughput screening (HTS) and drug discovery microplate reader applications. Other common applications include:
- Protein-protein interaction studies
- Biomarker detection
Comparison of detection modes in multimode plate readers
| Detection mode | Principle | Benefits and features | Common assays and applications |
|---|---|---|---|
| Absorbance | Measures the amount of light absorbed by a sample | •Cost-effective •Robust assays | •Protein quantitation •Nucleic acid quantitation •Cell viability •ELISA |
| Turbidimetry | Measures the light scattering of a sample | •Cost-effective •Robust assays | Bacterial growth curves |
| Fluorescence | Measures the emission of a fluorescent sample upon excitation | •High sensitivity •Wide dynamic range •Multiplexing | •Nucleic acid quantitation •Cell viability •Calcium flux assays •Reporter gene assays (e.g. GFP, YFP) |
| Luminescence | Measures the light produced in a chemical or biochemical reaction | •Highest sensitivity •Does not require excitation light | •Cell viability/ATP detection •Reporter gene assays •High-throughput screening |
| Time-resolved fluorescence | Measures delayed fluorescence emission of molecules with long fluorescence lifetimes | •High sensitivity •Time delay reduces fluorescence background | •Interaction studies •Post-translational modification studies •Drug discovery |
| Alpha detection | Measures light emission when beads are in close proximity | •Homogenous assay •Does not require washing •Excitation higher than emission eliminates fluorescence background | •High-throughput screening •Drug discovery •Interaction studies •Biomarker detection |
Thermo Scientific microplate reader options
Thermo Scientific offers microplate readers that utilize various detection modes. The Multiskan FC Microplate Photometer and Multiskan SkyHigh Microplate Spectrophotometer are both capable of performing absorbance and turbidimetric assays, while the Varioskan ALF and Varioskan LUX Multimode Microplate Readers can be used for a variety of detection modes including absorbance, turbidimetry, fluorescence, and luminescence. The Varioskan LUX is an advanced multimode plate reader that is also compatible with the specialized techniques time-resolved fluorescence and AlphaScreen/AlphaLISA detection.
Microplate readers use either filters or monochromators to isolate specific wavelengths of light for illumination and detection in the various detection modes. Filters allow a certain bandwidth of light to pass through to and from samples, while monochromators separate light into specific wavelengths for sample illumination and detection. Read the blog “Monochromator or Filter-Based Plate Reader? How to Choose” to learn more about the benefits and uses of monochromator- and filter-based plate readers.
| Plate Reader Model | Detection Modes |
|---|---|
 Multiskan FC | Absorbance, turbidimetry |
 Multiskan SkyHigh | Absorbance, turbidimetry |
 Varioskan ALF | Absorbance, turbidimetry, fluorescence, luminescence |
 Varioskan LUX | Absorbance, turbidimetry, fluorescence. Optional: time-resolved fluorescence (TRF), luminescence, AlphaScreen/AlphaLISA |
More tools and resources on microplate readers
- View our Guide to Microplate Readers
- Select Thermo Scientific microplate reader models
- Watch on demand: Strategies for Effective Microplate Reader Utilization
- Browse our Microplate Instruments, Assays, and Accessories Guide
- Explore our microplate assays and fluorescence microplate assays
Want to read more articles like this? Subscribe to Connect to Science.
##
© 2025 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
For Research Use Only. Not for use in diagnostic procedures.
Leave a Reply