This is the next post in our immuno-oncology blog series. Start from the beginning here or follow along as we discuss the applications and challenges regarding chimeric antigen receptor (CAR) T cells.
In the previous post, we described the process for creating chimeric antigen receptor (CAR) T cells. This novel application represents innovative therapeutic promise but there is much biological complexity remaining to be unlocked. At present, only two CAR T cell treatments are approved for clinical use, tisagenlecleucel (Kymriah™) and axicabtagene ciloleucel (Yescarta™), both against severe leukemia that has persisted or relapsed after other treatments.
Achieving clinical success with these CAR T cell therapies was easier, because blood cancers do not form solid tumors, which are an obstacle to numerous cancer interventions. Furthermore, since the antigens that define various forms of leukemia are extremely well understood, generating appropriate chimeric T cells for each host was much easier and less haphazard. That said, researchers still remain interested in CAR T cell therapy’s promise against solid tumors, and the technique has shown some success against melanoma lesions in recent trials.
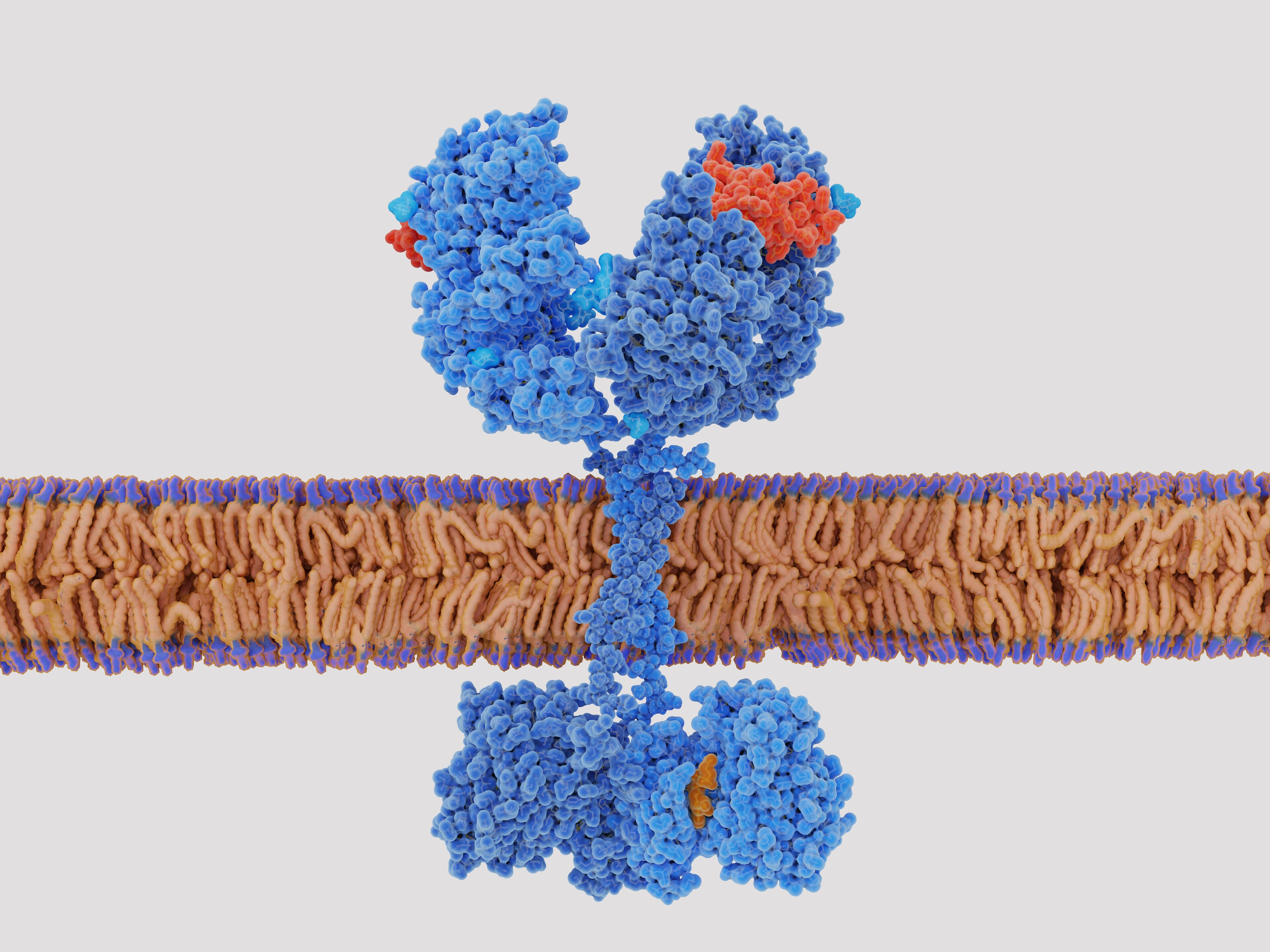
A major limitation of CAR T cell therapy is that identifying an antigen, deriving an antibody sequence, and creating the genetic engineering tools to introduce that sequence into a T cell together take a very long time. This series of steps is necessary to make this therapy as personalized as it is, but also means that the span between determining that CAR T cell therapy is needed and administering it is measured in weeks or months. To reduce this time, some researchers are working on “off-the-shelf” versions of CAR cell lines that can be administered much as monoclonal antibodies are today. These would target common cancer antigens that do not differ much between hosts. Reducing the personalization of the cell line increases the chances of an adverse reaction, but further engineering, discussed below, can bring that risk back down, in exchange for dramatically reducing the time required to use CAR T cell therapy.
Relatedly, part of the power of CAR T cell therapy is that it generally does not need to be repeated. The chimeric T cells can multiply on their own, the same as any other T cells, and thus their anti-cancer properties sustain themselves indefinitely. They are, in this way, more like an implanted medical device than a drug. Unfortunately, this means that any error in their creation likewise becomes an enduring part of the host. If a CAR T cell line targets a protein that is expressed in the host’s healthy cells, it will damage those cells just as readily as it attacks the cancer it was designed to fight.
Some cancers leave few options other than taking this risk, because their most pronounced and most easily targeted antigens are proteins that they overexpress rather than totally unique targets. This risk is likewise heightened with “off-the-shelf” CAR preparations, since these are not engineered to match the host’s proteins and so may show cross-reactivity. A host whose CAR T cells are attacking healthy tissue would have to take a lifetime regimen of immunosuppressants once their cancer is dealt with, and possibly even before that, to protect themselves from their chimeric cell line.
Alternative formulations make CAR T cell lines a temporary rather than permanent undertaking. If a yeast plasmid is used instead of CRISPR or a virus, the T cell will still express its chimeric receptor, but the plasmid will not transmit as the cell divides, slowly depleting the chimeric cell population in the host. Alternatively, a reproductive checkpoint also engineered into the chimeric cell can prevent it from reproducing further after a fixed number of cell divisions. If the cancer outlives this timed treatment, the host would have to receive another round, but that could be preferable to incurring the risk of a new autoimmune disease.
CAR T cell therapy is the culmination of decades of research in oncology, immunology, and genetics, using the newest techniques to try something never before attempted. There are yet complex challenges ahead and so resolving those challenges will be the work of tomorrow.
To learn more, visit our one-stop Immuno-oncology Hub or get our I-O Resource Guide.
For Research Use Only. Not for use in diagnostic procedures.


Leave a Reply