
For anyone designing or troubleshooting a western blot experiment, the question “PVDF vs nitrocellulose?” may not be top of mind beside considerations of antibody choice, transfer type, or detection method.
But transfer membrane selection can make a big difference in sensitivity, dynamic range, and overall quality of results. It’s a process step that deserves your careful thought – especially if you are aiming for journal submission.
In this guide, we’ll focus on choosing (and handling) an excellent membrane for your western blotting needs.
Table of contents
- How to choose a membrane like PVDF or nitrocellulose
- Pros and cons of nitrocellulose membranes
- Pros and cons of PVDF membranes
- Pros and cons of low fluorescence PVDF membranes
- PVDF or nitrocellulose: which is better?
- Common questions about PVDF and nitrocellulose membranes
- How to store a western blot membrane after transfer: is the process different for nitrocellulose vs PVDF?
- What are the differences in activation steps between nitrocellulose and PVDF membranes?
- What happens if a PVDF membrane dries out?
- I have an iBlot 3 or iBlot 2 gel transfer device. What type of membranes do I need?
- Are transfer times different between PVDF and nitrocellulose?
- More resources about PVDF and nitrocellulose membranes
How to choose a membrane like PVDF or nitrocellulose
Western transfer membranes are essential in Western blotting because they serve as a stable platform to immobilize proteins after electrophoretic separation. Once proteins are transferred from a gel onto the membrane, researchers can use specific antibodies to detect and quantify proteins of interest.
The membranes are engineered to have high protein-binding capacities and are compatible with various detection methods, such as chemiluminescence, fluorescence, and colorimetric assays, which ultimately contribute to the sensitivity and accuracy of the analysis.
The two main transfer membrane options in western blotting are PVDF and nitrocellulose. Both produce similar, reliable results across many targets, but each option offers distinct advantages in particular scenarios.
So, how do you choose?
The optimal membrane may depend on your protein target
Both nitrocellulose and PVDF offer strong binding affinity for most proteins and either membrane can often be used to achieve similar results (Figure 1). However, in some cases, a particular protein of interest will have chemical properties (e.g., hydrophobicity) that will complement the binding mechanism(s) of one type of membrane when compared to the other, resulting in increased membrane affinity and an apparent preference for that membrane type.
In these cases, membrane choice may have a significant impact on western blot results. A poor protein-membrane interaction may result in the protein releasing from the membrane during incubation or wash steps. In severe cases, it may prevent the protein from binding to the membrane altogether. If you are having trouble detecting your protein of interest, it may be worth simply trying a different membrane type as a starting point.
Detection method also matters – chemiluminescent or fluorescent?
PVDF and nitrocellulose membranes perform similarly in chemiluminescent applications. For fluorescent detection methods, however, there are key performance differences between membrane types that may influence your choice. Low fluorescence PVDF is a versatile option that does well across all detection methods but offers exceptional fluorescent performance.
Some users may have membrane handling preferences, too.
The ease of membrane handling may be an important factor for users who want to minimize the number of process steps and amount of hands-on time. Some membranes require activation steps before use to help ensure optimal binding. Durability may be important for users who need to strip and reprobe their blot several times.
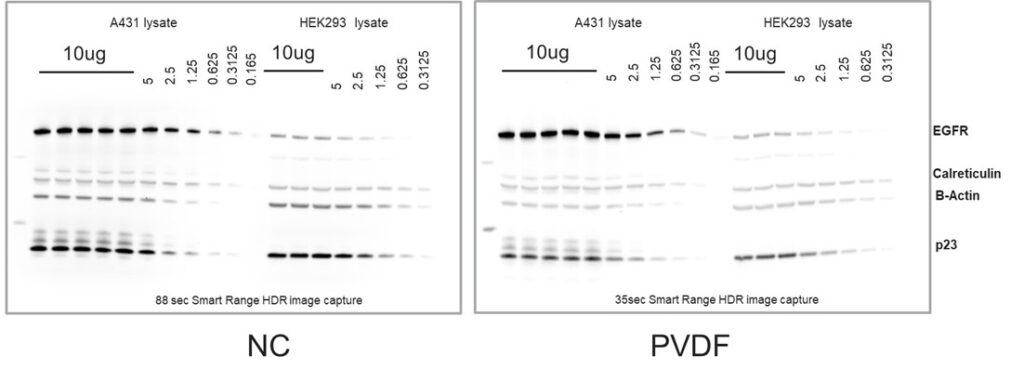
PVDF and nitrocellulose transfer membranes can both be effective western blotting tools in the right circumstances. Let’s dive into the pros and cons of each membrane type.
Pros and cons of nitrocellulose membranes
Harry Towbin used a nitrocellulose membrane when he first developed the western blot technique in 1979, and this method remains a highly published standard today.
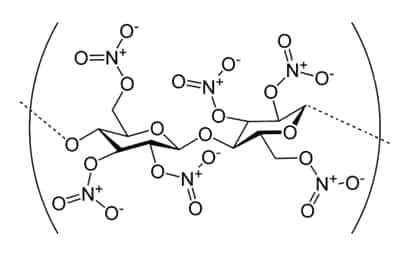
Nitrocellulose membranes are produced via nitration, where cellulose reacts with nitric and sulfuric acids – swapping in nitro groups for hydroxyl. These membranes can bind and immobilize a wide variety of proteins through a combination of hydrogen bonding and nitrogen dipole, ionic, and hydrophobic interactions.
Pros
- Suitable for a wide range of protein types
- No activation step necessary for protein transfer
Cons
- More prone to tearing during handling than PVDF
- Not compatible with some solvents or highly acidic conditions
- Stricter regulation in some regions due to flammability
- Not optimal for fluorescent western blotting due to autofluorescence, particularly at shorter wavelengths
Pros and cons of PVDF membranes
Polyvinylidene difluoride (PVDF) membranes can be manufactured either as a homopolymer of vinylidene difluoride or as a copolymer with other additives. This results in a robust and stable membrane that binds proteins through hydrophobic interactions. In addition, PVDF membranes are highly stable both physically and chemically, making them easy to handle and compatible with most western blotting workflows. These properties make PVDF a reliable choice favored by many researchers.
However, unlike nitrocellulose, PVDF membranes must be activated before use. Although brief, this means an additional step in an already time-intensive experiment like a western blot.
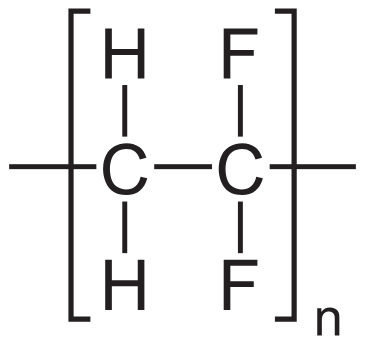
Pros
- Suitable for a wide range of protein types
- Physically and chemically stable, less prone to tearing
Cons
- Requires activation step / wetting before use, which involves methanol or ethanol solutions
- Higher levels of autofluorescence than nitrocellulose, which can severely impact usable fluorescent channels, detection limits, and accuracy during fluorescent experiments
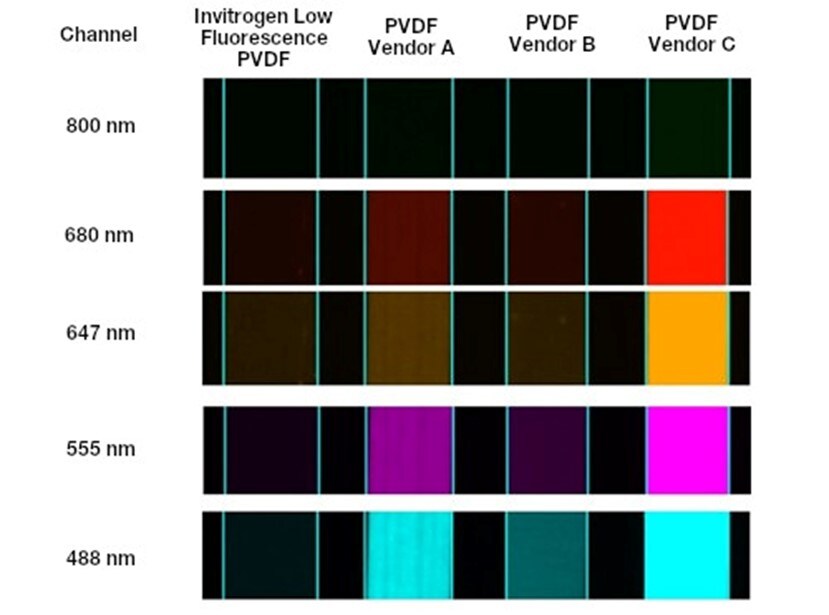
Pros and cons of low fluorescence PVDF membranes
Low fluorescence PVDF membranes are often used in fluorescent western blotting or alongside fluorescent total protein normalization methods to maximize signal-to-noise.
They are designed to reduce sources of autofluorescence—such as raw materials and impurities—resulting in a lower fluorescent background compared to both nitrocellulose and standard PVDF membranes (Figure 3).
In addition to improved sensitivity, the lower fluorescent background enables the use of a wider range of fluorescent imaging channels, allowing researchers to extract significantly more information from each western blot through multiplexing. Finally, many low fluorescence PVDF membranes perform similarly to standard PVDF membranes in chemiluminescent applications, making them the most versatile membrane option.
Pros
- Suitable for a wide range of protein types
- Perform similarly to standard PVDF membranes in chemiluminescent applications
- Designed to reduce autofluorescence, making them ideal for fluorescent detection
Cons
- Requires activation step / wetting before use, which involves methanol or ethanol solutions

PVDF or nitrocellulose: which is better?
In summary, both nitrocellulose and PVDF membranes are effective for western blotting, with each having distinct advantages and disadvantages.
- Nitrocellulose membranes don’t require activation but may be more prone to tearing.
- PVDF membranes, while needing activation, offer high durability and chemical compatibility.
- Low fluorescence PVDF, the most versatile membrane option, performs well across all detection methods but offers excellent fluorescent performance.
While the most effective way to identify the optimal membrane for your specific protein of interest is to test how your protein binds to each membrane type, this can be tedious and time consuming. So if durability and versatility are priorities – particularly for fluorescent detection – low fluorescence PVDF membranes are generally the right choice.
The table below summarizes the key differences between nitrocellulose, PVDF, and low fluorescence PVDF membranes.
| Property | Nitrocellulose | PVDF | Low Fluorescence PVDF |
|---|---|---|---|
| Chemiluminescent detection | +++ | +++ | +++ |
| Fluorescent detection | ++ | + | +++ |
| Total protein normalization | ++ | + | +++ |
| Requires activation? | No | Yes | Yes |
| Binding mechanism | Nitrogen dipole, H-bond, ionic, and hydrophobic interactions | Hydrophobic interactions | Hydrophobic interactions |
Common questions about PVDF and nitrocellulose membranes
Below, our R&D experts share their responses to some of the most common membrane questions they hear from researchers.

Blotting tips for new and experienced users
Master fluorescent western blotting as a new user or learn how to maximize the technique with multiplexing with this pair of application notes.
How to store a western blot membrane after transfer: is the process different for nitrocellulose vs PVDF?
Ideally, you should proceed directly to immunodetection immediately after transfer. But if you need to store your membrane for immunoprobing later, the recommended steps are as follows:
- Rinse: Rinse the membrane briefly in distilled water to remove any residual transfer buffer.
- Drying: Place the membrane on clean filter paper and allow the membrane to air dry completely. Nitrocellulose and PVDF membranes are stable when dried and can be stored for extended periods.
- Storage: Store the dried membranes in a clean, dry container or between sheets of filter paper at room temperature. Ensure that the membrane is kept flat to avoid creasing.
Dried PVDF membrane needs to be rehydrated in 100% methanol or ethanol before blocking and probing with the primary antibody.
What are the differences in activation steps between nitrocellulose and PVDF membranes?
PVDF membranes require pre-activation by immersing them in 100% methanol or ethanol for 3 minutes followed by a quick rinse in diH2O before use. This activation step is necessary due to the highly hydrophobic characteristics of PVDF which prevent it from readily interacting with water.
During the activation step, methanol or ethanol serves as a wetting agent, breaking surface tension between the nonpolar membrane and polar buffer solution and allowing them to interact. Methanol can also enhance the hydrophobic interactions of the membrane allowing the transferred protein to tightly bind to the membrane.
In contrast, nitrocellulose membranes are generally ready for use after a simple wetting step in buffer or water, as they do not need methanol treatment. These differences mean that PVDF membranes involve an extra activation step, while nitrocellulose membranes are more straightforward to prepare.
What happens if a PVDF membrane dries out?
If the PVDF membrane dries out prior to blocking, the membrane can be hydrated according to the procedure above before proceeding to the blocking step. Generally, this will not impact protein binding. It is recommended to avoid drying immediately prior to detection with chemiluminescent substrate. If using a fluorescent detection method, most blots can typically be imaged dry without re-wetting, but some fluorescent dyes are prone to degradation which can be accelerated when membrane drying occurs.
I have an iBlot 3 or iBlot 2 gel transfer device. What type of membranes do I need?
The iBlot 3 system uses nitrocellulose, PVDF, and Low Fluroescence PVDF membranes for protein transfer while the iBlot 2 uses only nitrocellulose and PVDF. iBlot Transfer Stacks incorporate these membranes directly in the stack or packaged on the side, which helps provide maximum convenience while maintaining excellent transfer efficiency.
Are transfer times different between PVDF and nitrocellulose?
Generally, the transfer times for PVDF and nitrocellulose membranes are similar under standard conditions. However, slight adjustments in protocol may be needed depending on factors such as protein size, gel composition, and the transfer apparatus.
More resources about PVDF and nitrocellulose membranes
You can learn more about the western transfer process and beyond at thermofisher.com:
- Overview: Western Blotting Transfer Methods
- Application Notes – Basic Fluorescent Western Technique & Multiplexing
- Overview: Fluorescent Western Blotting
##
© 2025 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
For Research Use Only. Not for use in diagnostic procedures.



Leave a Reply