In the fast-paced world of biopharmaceutical manufacturing, ensuring product quality and safety is paramount. One of the critical aspects of maintaining this product integrity is mycoplasma testing. Mycoplasma contamination poses a significant threat to therapeutic efficacy and safety, highlighting the need for robust and sensitive testing solutions. The significance of routine testing, not only as part of in-process testing, is a key element in lot-release procedures. Let’s delve into the significance of mycoplasma testing, explore the features and benefits of PCR-based testing, and how this advancement elevates the standards of biotherapeutic, and specifically cell and gene therapy, manufacturing.
The Mycoplasma Menace
Mycoplasma contamination is a recurring concern in biotherapeutics manufacturing due to its potential to devastate product quality and patient safety. Mycoplasmas are the smallest self-replicating microorganisms in existence. These tiny bacteria lack a cell wall and are difficult to remove from processes due to their small size. Mycoplasma can infect a wide range of host cells and media, making them a sneaky, silent threat in manufacturing. Unlike with other microbial contamination that cause cloudiness in growth media, mycoplasma contamination is not visible. Not only can mycoplasma undermine the desired therapeutic effect, but they can also trigger immune responses in a patient population that is already battling other syndromes and diseases. So, rigorous mycoplasma testing is essential to maintain the highest standards in cellular product manufacturing.
What’s so important about in-process and lot-release testing?
In-process mycoplasma testing can be thought of as a sentry guarding a gate. The test watches over key points in the bioproduction process, from raw material and cell bank testing through lot-release. By implementing this testing at critical junctures, you can catch mycoplasma contamination early, enabling prompt intervention. For example, in a monoclonal antibody (mAb) production workflow, as shown in figure 1, there are several points to test for mycoplasma. This not only reduces the risk of downstream product issues, but also can help save significant costs associated with disposing of a contaminated product and shutting down a facility to sterilize it.
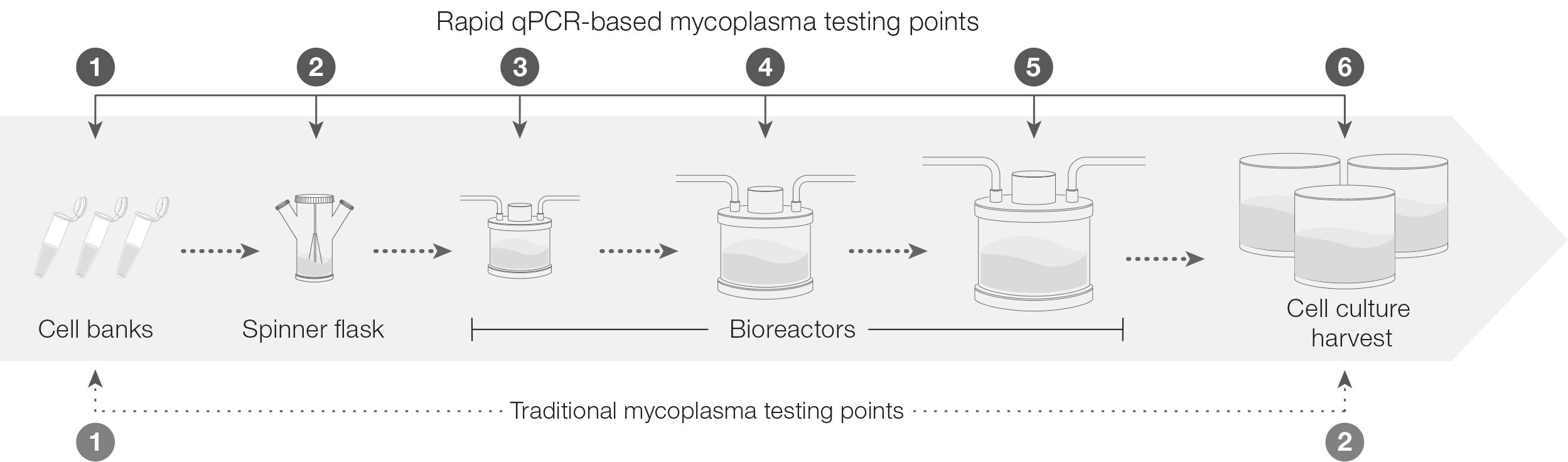
Figure 1: Testing points within the therapeutic bioproduction workflow
Modern mycoplasma testing methods are highly sensitive, specific, and efficient. This makes it easier than ever to help ensure your products are free from these invisible threats. Enter the Applied Biosystems™ MycoSEQ™ Mycoplasma Detection System. This detection solution offers several key features, including:
- Superior sensitivity: The Applied Biosystems™ MycoSEQ™ Plus Detection System employs advanced DNA amplification and detection techniques via TaqMan chemistry. This technology helps enable an extra layer of confidence in cell therapy manufacturing. The kit meets or exceeds the regulatory threshold of 10 CFU/mL (equivalent to 10 genome copies/mL).
- Comprehensive coverage: The technology’s comprehensive panel of oligos were designed to detect more than 200 mycoplasma species (based on in silico analysis), including Hemoplasma (i.e., wenyonii), Mesoplasma and Ureaplasma groups. This is crucial as a wide variety of mycoplasma strains can infect cell lines and media.
- Rapid time to detection: Time is of the essence for any biotherapeutic—and especially for cell and gene therapies. Leveraging real-time PCR, the MycoSEQ Plus Kit significantly reduces the time to results in hours. The traditional culture-based methods typically have a 28-day turnaround. With this time savings, you can make informed decisions quickly and reduce potential downtime. This can also help with keeping the pace with the overall production process.
- High specificity: The MycoSEQ Plus detection kit is highly specific to mycoplasma. It has been shown to not cross-react with common bacterial contaminants (or bacteria with close genetic phylogeny), cell-line DNA, or growth media additives.
- Reduced false positives/negatives: The discriminatory internal positive control is a proprietary mycoplasma surrogate that can be used as extraction and positive PCR controls. With this control, you eliminate the need to use live mycoplasma for these tasks. The positive control can help uncover a potential cross-contamination event and can enable you to figure out which actions are needed.
The features of the MycoSEQ Plus Detection System has many benefits, including:
- Enhanced product safety: By delivering highly accurate results, the MycoSEQ Plus Kit helps ensure that your biotherapeutic is free from mycoplasma contamination.
- Cost-efficiency: MycoSEQ Plus assays enables you to get more done. Optimize resource allocation by reducing the need to outsource lengthy testing and enables cost-effectiveness for batch testing and scale-up.
- Regulatory compliance: Meeting regulatory guidelines is crucial for all biotherapeutic products. The MycoSEQ Plus kit assists in complying with these guidelines, as they relate to mycoplasma testing.
In the world of biopharmaceutical manufacturing, mycoplasma testing is not just a regulatory checkbox. It’s a cornerstone of quality control and patient safety. By integrating mycoplasma testing into your raw materials, cell bank, in-process and lot-release protocols, you’re not just complying with regulations. Rigorous testing also demonstrates a commitment to the highest standards of quality and ethics. The MycoSEQ Plus Kit leverages advanced detection technology to bolster sensitivity, efficiency, and reliability. The biotechnology landscape is continually evolving. Innovations like the MycoSEQ Plus Detection System, can help illuminate the path forward towards safe, effective, and accessible therapies.
Visit our mycoplasma detection solution page to learn more.
Recommended resources:
- Webinar: Learn how to simplify and speed up your mycoplasma detection
- Product bulletin: MycoSEQ Mycoplasma Detection Kits
- Infographic: Rapid Mycoplasma testing: Meeting regulatory requirements with confidence
For Research Use Only. Not for use in diagnostic procedures.
Leave a Reply