Rapid identification of opioids
As is all too common, police arrive at the scene of a drug overdose. While the victim is treated with Naloxone to reverse the effects of a suspected opioid compound, a clear plastic bag is collected, evidenced and sent to police headquarters. While state and local crime labs are backed up with overwhelming caseloads, a rapid identification of the compound found in the plastic bag would be helpful to law enforcement to have a better understanding of the drug. It’s likely that more of this drug is circulating on the street.
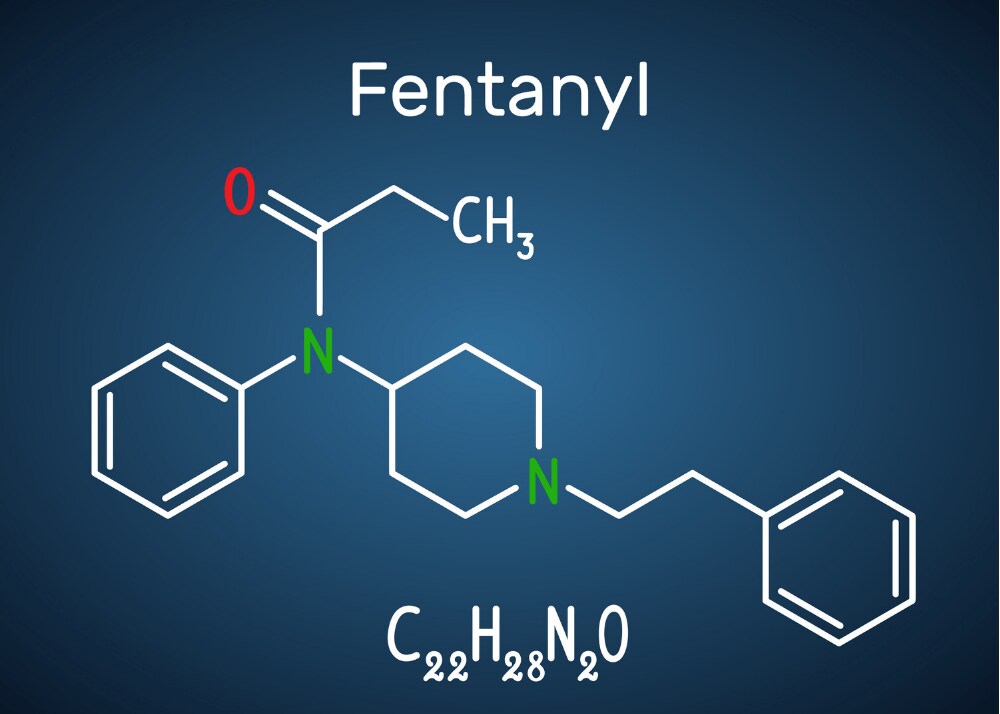
Fentanyl molecular structure
The opioid epidemic began with the overuse and abuse of prescription pain medication. When the federal government began imposing hundreds of millions of dollars of fines on the largest drug distributors and pharmacies between 2008 and 2015, the supply began to tighten and pill addicts turned to heroin, triggering the second wave of the epidemic.
Increased heroin use ultimately led to the third wave of the epidemic, as Mexican drug cartels began blending their heroin with fentanyl, a deadly synthetic opioid. Drug dealers in the United States also began selling fentanyl, leading to more than 67,000 overdose deaths from fentanyl from 2013 to 2017.
What is fentanyl?
Fentanyl was created in 1959 as an intravenous surgical analgesic. It is 50 to 100 times more powerful than morphine. As an opioid drug, fentanyl is sometimes used deliberately by people who use other opioid drugs, such as heroin and prescription painkillers. But due to its potency, it has made its way into many other drugs that people use recreationally. However not all drugs labeled as “fentanyl” exhibit the same potency–which leads to a major problem.
Drug analogs are drugs that are developed to imitate a particular drug. It is important to note that they are not identical. Illicit drug manufacturers create “designer drugs” that could not be listed as illegal or controlled drugs until they were recognized. However, any drug that was structurally similar to a controlled drug is also illegal, and drug manufacturers continue to develop new drug analogs to stay in business.
The potency of fentanyl analogs ranges from half as potent as fentanyl to several times more potent. Given the pharmacological strength and therefore, the cost-effectiveness of using fentanyl and its analogs and derivatives as a cutting agent, the risk of fentanyl being mixed with other drugs is higher than ever. This has led to an unprecedented number of people dying from opioid overdoses—people who never thought they were at risk because they did not knowingly take opioids.
Fentanyl analogs
Whereas fentanyl is 50 to 100 times more powerful than morphine, the analog carfentanil is 10,000 times more powerful than morphine. In fact, it was never even intended for use by humans but was only intended to treat large animals many times our size. Another analog, butyrfentanyl, has no current legitimate clinical applications; however, it is widely sold as a designer drug.
Similar to butyrfentanyl, isobutyrylfentanyl is one quarter as powerful as fentanyl. It was one of the first analogs to be sold through the Internet as a designer drug and was one of the earliest of the “new wave” of fentanyl derivatives to appear, and was reported in Europe for the first time in December 2012. Another analog, 3-Methylfentanyl (3-MF) is one of the most potent opioids on the street, estimated to be between 400 and 6000 times stronger than morphine. Its potency depends on which isomer is used, with the cis- isomers being the more potent ones.
In organic chemistry, isomers are molecules with the same molecular formula (i.e., the same number of atoms of each element), but different structural or spatial arrangements of the atoms within the molecule. The reason there are such a colossal number of organic compounds – more than 10 million – is in part due to isomerism.
Identification of fentanyl
The unequivocal identification of the suspected fentanyl analog is complicated by the fact that each drug can be a unique isomeric compound. Due to their structural similarity, some isomers have identical accurate mass, electron impact, and electrospray ionization fragmentation patterns, as well as close retention times on most gas and liquid chromatography methods. This eliminates several laboratory techniques as a reliable means of determining the specific structure of compound in question. Also, lab methods may require using solvents to prepare the sample for analysis, destroying it for storage or complementary analysis.
Raman spectroscopy for fentanyl identification
Raman spectroscopy, based on inelastic light scattering, allows for rapid, inexpensive and nondestructive analysis in forensic science. Quantitative Raman spectroscopy is used to determine the actual drug concentrations in street cocaine and crack rocks and to identify possible adulterants in these samples for forensic toxicology and criminalistics. Developments in Raman spectrometers (portable instruments and new excitation wavelengths) and advancements in data analysis offer exciting opportunities for new applications of Raman spectroscopy in the identification and quantification of drugs of abuse, including investigations conducted immediately at the scene of a crime. While portable Raman instruments, such as the Thermo Scientific™ TruNarc™ Handheld Narcotics Analyzer, are extremely useful at crime scenes for rapid identification of substances, these instruments may not have the sensitivity to discriminate between fentanyl analog isomers.
Fourier-transform Raman spectroscopy for isomer identification
Fourier-transform (FT) Raman spectroscopy is a common lab bench technique capable of determining analog isomers. The technique is non-destructive, and ideal for sampling through plastic bags or glass, greatly reducing the risk of lab personnel’s accidental exposure to high potency drugs. Being nondestructive, the technique maintains the custody chain from its seizure until the sample is placed in the evidence file.
Recently scientists from Thermo Fisher Scientific used FT-Raman spectroscopy to analyze a pair of constitutional isomers: butyryl fentanyl and isobutyryl fentanyl, and also a pair of geometric isomers: cis- and trans- 3-methyl fentanyl. What they found is that the minute structural variations between isomers resulted in significant differences in the Raman spectra, allowing for positive identification and effective discrimination of the isomers.
Learn more about how Raman spectroscopy is used in drug identification.
Want to speak to someone? Need a quote? Want a demo? |
Opt in for email communications on this and other topics. |
Leave a Reply