Search Thermo Fisher Scientific
Bioprocessing Learning Center

Biologics improve the quality of life for humans and animals worldwide. Quality requirements, global supply needs and manufacturing efficiency are critical to biopharmaceutical manufacturers. Thermo Fisher Scientific brings decades of bioprocessing experience and knowledge, a collaborative customer-focused approach, performance-tested and reliable technologies, and products and services to drive your success.
Bioprocessing featured resources
Article
Innovative Downstream Purification Solutions for Viral Vectors
Over the past decade, gene therapy applications and their importance in the biopharmaceutical industry have been increasing. Gene therapies promise versatile treatment options that could revolutionize and transform medicine.
White paper
Introduction to mass transfer
in single-use bioreactors
Mass transfer in Thermo Scientific HyPerforma Single-Use Bioreactors (S.U.B.s) is critical to the growth of cells in culture, yet is often a misunderstood principle due to its complexity. A basic understanding of mass transfer and its underlying principles is essential to single-use bioreactor design, determining bioreactor operating parameters, and optimizing culture conditions. This paper aims to elucidate the standard mass transfer model and details how the application of critical mass transfer principles to the design and operation of the HyPerforma S.U.B. helps to achieve optimal mass transfer performance for cell culture operations.
White paper
Gas Analysis Mass Spectrometry Applications in Fermentation and Cell Culture Processes
Fermentation is the term used by microbiologists to describe the production of a compound by means of the mass culture of a microorganism. This product can either be the cell itself (biomass production), the microorganism’s own metabolite or a foreign product.
Advantage of antibody based selectivity in the purification of biologics
Advances in biotherapeutics are generating an increasing range of complex molecules that present unique and often complex purification challenges. By taking advantage of antibody-based selectivity, Camelid heavy-chain antibody fragments (VHHs) have proven to be a reliable immuno affinity chromatography (IAC) solution in the downstream process of biologics. Thermo Scientific CaptureSelect affinity products and analytical tools are developed for the discovery and manufacturing of even the most demanding biotherapeutics. The affinity resins provide high target purity in a single step, independent of feedstock.
Over the past decade, gene therapy applications and their importance in the biopharmaceutical industry have been increasing. Vectors centered on the nonpathogenic adeno-associated virus (AAV) have emerged as the vector of choice for many therapies. Although the biopharmaceutical industry is primed to purify viral vectors on a scale suitable for clinical supply or orphan indications, it is still working toward generating industrialized platform technologies that will maximize productivity and enable efficient, simple, and inexpensive purification of biologically active viral vectors for large-scale commercial manufacturing. Thermo Fisher Scientific has developed downstream purification solutions enabling the scale-up of these viral vectors.
Single-step affinity purification of antibodies and antibody-fragments by Kappa light chain binding
With the development of novel biotherapeutic antibody formats, such as trifunctional and bi-specific monoclonal antibodies or antibody fragments, new purification challenges in the downstream process of these molecules arise. Thermo Scientific CaptureSelect antibody subdomain-specific affinity products are developed for the discovery and manufacturing of therapeutic antibodies and antibody fragments. The affinity resins provide high target purity in a single step, independent of feedstock. Here we show the performance of the CaptureSelect KappaXP affinity resin, a next generation Kappa Light chain binder, with improved dynamic binding capacity and mild elution properties, in comparison with alternative products.
Accelerating antibody drug development with subdomain-specific affinity ligands
With the development of novel bio-therapeutic antibody formats, such as trifunctional and bi-specific monoclonal antibodies or antibody fragments, new purification challenges in the downstream process of these molecules arise. Thermo Scientific CaptureSelect antibody subdomain-specific affinity products and analytical tools are developed for the discovery and manufacturing of therapeutic antibodies and antibody fragments. The affinity resins provide high target purity in a single step, independent of feedstock.
Advantage of antibody-based selectivity in the purification of biologics
Advances in biotherapeutics are generating an increasing range of complex molecules that present unique and often complex purification challenges. By taking advantage of antibody based selectivity, Camelid heavy-chain antibody fragments (VHHs) have proven to be a reliable immuno affinity chromatography (IAC) solution in the downstream process of biologics. Thermo Scientific CaptureSelect affinity products and analytical tools are developed for the discovery and manufacturing of even the most demanding biotherapeutics. The affinity resins provide high target purity in a single step, independent of feedstock.
Automated foam control in Single-Use Bioreactors using a single-use foam probe
Cell culture and fermentation has the potential to generate foam in great quantity in stirred-tank reactors. To reduce the need for constant supervision, manual intervention, and excessive chemical anti-foam usage, an automated foam probe is proposed. A foam detection system was designed to provide accurate and consistent results. It was designed to resist forces applied from manufacturing, shipping, and BPC installation, and to resist fouling in culture. The system was tested to automatically manage foam in several cell culture applications and was compared to time-based anti-foam addition strategies. Comparisons included viable cell density and amount of anti-foam used.
Featured bioprocessing videos
 Learn how you can achieve optimal bioprocessing outcomes
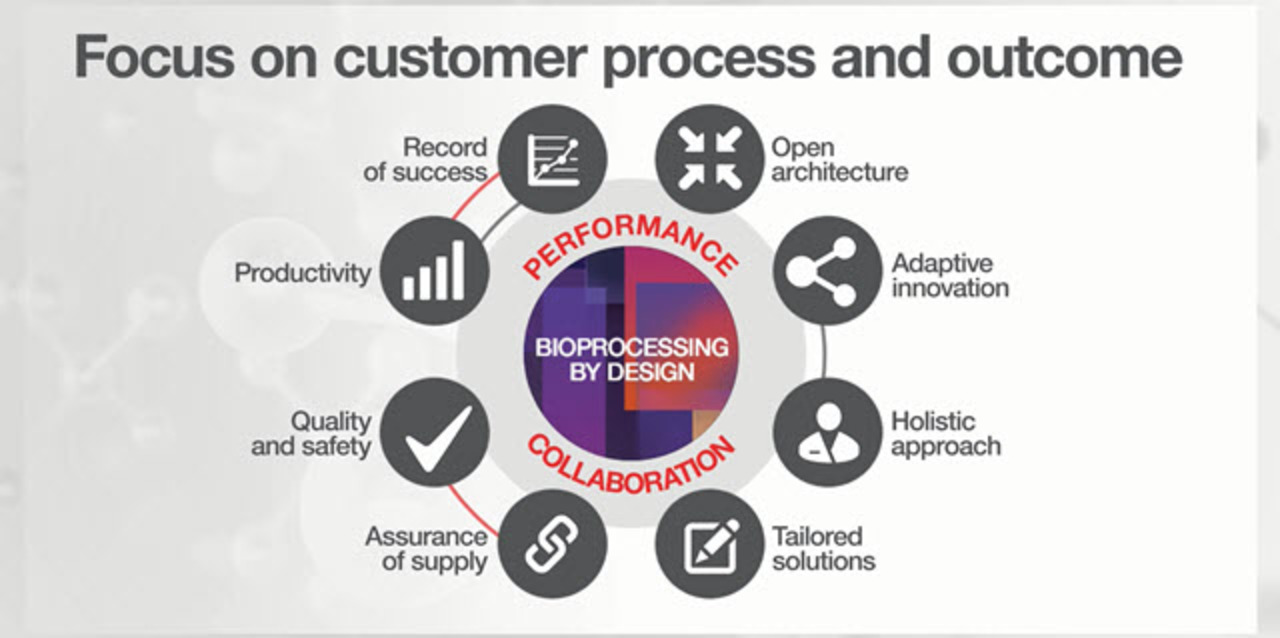
Learn how you can achieve optimal bioprocessing outcomesWith a proven portfolio of solutions that span discovery through large-scale commercial production, we bring process intensification across your upstream and downstream workflows, without compromising on quality.
 What Makes a “Good” Bioprocess?
What Makes a “Good” Bioprocess?The article introduces three main questions a manufacturer should ask themselves: 1) How do I simplify my process? 2) How do I ensure consistent performance? 3) How is upstream impacting downstream?
 Keys to Consistent Bioprocessing
Keys to Consistent BioprocessingThe development of a robust and consistent bioprocess requires a systematic and risk-based approach to identify the right process parameters and raw materials.
 Process Intensification - Getting More From Less
Process Intensification - Getting More From LessIntensifying or simplifying your bioprocess can mean more product, shorter manufacturing times, or lower costs –understanding what matters most is key to making the right decisions.
 The Upstream/Downstream Process Balancing Act
The Upstream/Downstream Process Balancing ActEarly collaboration and open communication between upstream and downstream are crucial to ensure a consistent end-to-end bioprocess.
Bioprocessing subtopics
Antibody and Cell Culture Information
The production of therapeutic antibodies and proteins is one of the fastest growing sectors of the pharmaceutical industry, where accuracy and consistency in bioprocessing can pose challenges.
Cellular and Stem Cell Information
Challenges in the production of cellular therapies are many, including safe navigation in a dynamic regulatory landscape, quality and consistency of processes, and safety and productivity, all in a cost-effective manner.
Vaccine Information
As the global demand for vaccine doses dramatically increases, the need for highly efficient manufacturing processes, collaborative technical resources and a global supply partnership between vaccine producers and their suppliers is now an absolute necessity.
Bioprocessing Resource Library
Access a targeted collection of scientific application notes, case studies, videos, webinars, and white papers for bioproduction.
Bioprocessing resources
Access a targeted collection of scientific application notes, case studies, posters, white papers and more for bioprocessing: