Search Thermo Fisher Scientific
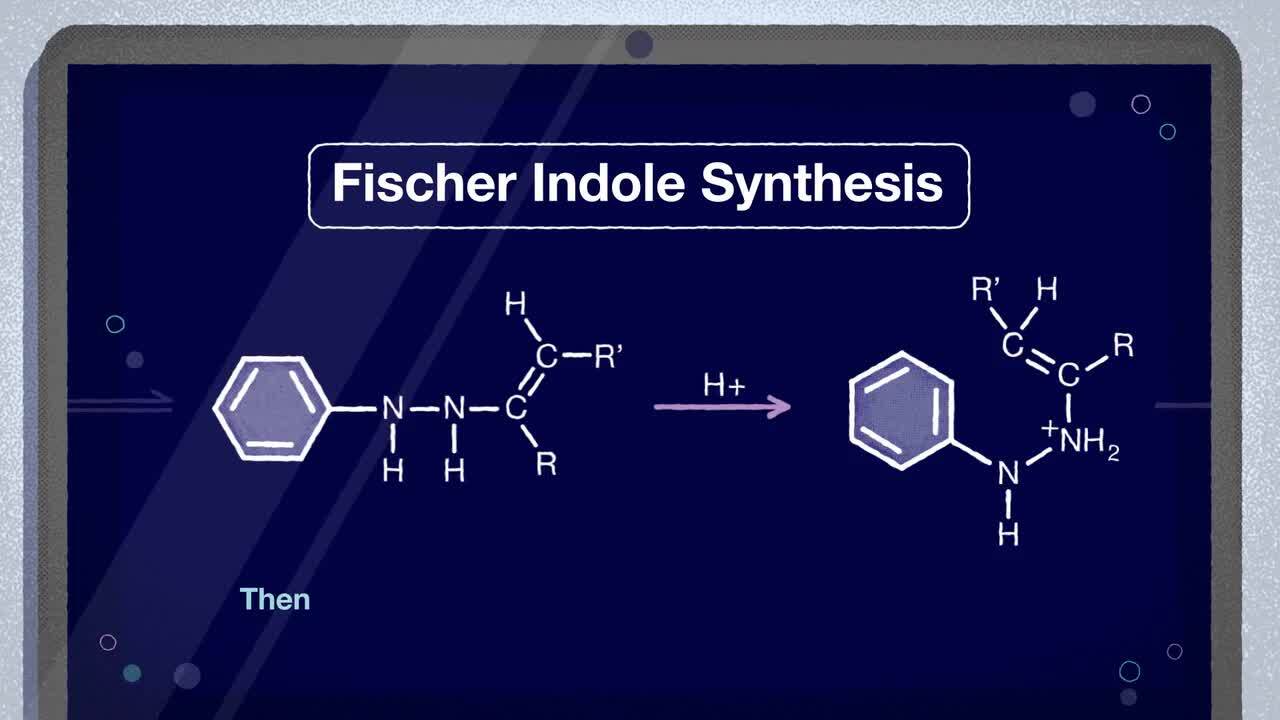
The Fischer Indole Syntheis is the most important method of preparing substituted indoles.

One of the oldest reactions in organic chemistry, the Fischer Indole synthesis is the condensation of a substituted phenylhydrazine and a carbonyl compound under acidic conditions to produce an aromatic heterocycle indole.
Fischer Indole Synthesis Mechanism
Watch our video to learn more about the Fischer Indole synthesis and its mechanisms.
History of the Fischer Indole Synthesis
In 1883, E. Fischer and F. Jourdan treated pyruvic acid 1-methylphenylhydrazone with alcoholic hydrogen chloride and generated 1-methylindole-2-carboxylic acid. Preparing indoles by heating the arylhydrazones of either aldehydes or ketones in the presence of a protic or Lewis acid is now known as the Fischer indole synthesis. Since its discovery, it has remained the most important method of preparing substituted indoles.
Main features of the Fischer indole synthesis:
- The indole formation can be carried out as a one-pot synthesis, as it is not necessary to isolate the intermediate arylhydrazones
- Unsymmetrical ketones give two region-isomeric 2,3-disubstituted indoles, with the region-selectivity dependent on acidity of the medium, substitution of the hydrazine, and steric effects
- 1,2-diketones can give both mono and bis-indoles, the mono-indoles usually forming with strong acid catalysts in refluxing alcohols
Other Heterocycle Formation Reactions
Other Heterocycle Formation Reactions include:
-
Hantzsch Dihydropyridine Synthesis -
Knorr Pyrrole Synthesis -
Pictet-Spengler Tetrahydroisoquinoline Synthesis -
Pomeranz-Fritsch Reaction
For other types of reactions, visit our Named Reactions page.
Acroseal Packaging
Chemical reactions often involve the use of air- and moisture-sensitive solvents, and pyrophoric or hazardous reagents. Our AcroSeal packaging is a packaging solution designed to enable safe handling of these types of materials which are used in a variety of research and development applications, including NMR analysis and studies in drug discovery, agrochemicals, flavors and fragrances, and more.
Watch our video for more information.