Search Thermo Fisher Scientific
Thermo Scientific Chemicals
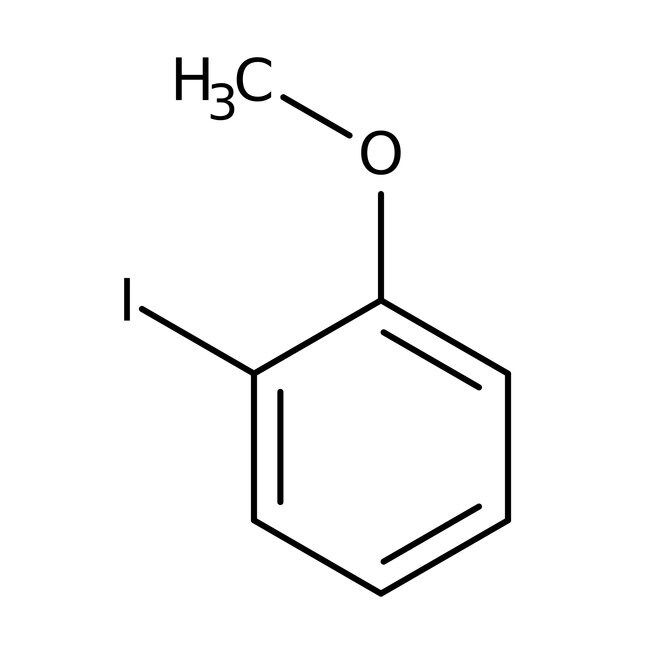
2-Iodoanisole, 99%, Thermo Scientific Chemicals
Catalog number A10150.14
also known as A10150-14
Price (USD)/ Each
42.65
Online exclusive
47.30 Save 4.65 (10%)
-
Quantity:
25 g
Price (USD)/ Each
42.65
Online exclusive
47.30 Save 4.65 (10%)
2-Iodoanisole, 99%, Thermo Scientific Chemicals
Catalog numberA10150.14
Price (USD)/ Each
42.65
Online exclusive
47.30 Save 4.65 (10%)
-
Chemical Identifiers
CAS529-28-2
IUPAC Name1-iodo-2-methoxybenzene
Molecular FormulaC7H7IO
InChI KeyDVQWNQBEUKXONL-UHFFFAOYSA-N
SMILESCOC1=CC=CC=C1I
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear pale yellow to yellow
FormLiquid
Assay (GC)≥98.5%
Identification (FTIR)Conforms
Refractive Index1.6210-1.6240 @ 20?C
2-Iodoanisole has been used in palladium/copper-catalyzed synthesis of o-(1-alkynyl)anisoles.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Iodoanisole has been used in palladium/copper-catalyzed synthesis of o-(1-alkynyl)anisoles.
Solubility
Miscible with alcohol, chloroform and diethyl ether. Insoluble in water.
Notes
Light Sensitive, store in dark. Store in cool, dry conditions in well sealed container. Store away from oxidizing agent.
2-Iodoanisole has been used in palladium/copper-catalyzed synthesis of o-(1-alkynyl)anisoles.
Solubility
Miscible with alcohol, chloroform and diethyl ether. Insoluble in water.
Notes
Light Sensitive, store in dark. Store in cool, dry conditions in well sealed container. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- Dawei Yue et. al.Synthesis of 2,3-disubstituted benzo[b]furans by the palladium-catalyzed coupling of o-iodoanisoles and terminal alkynes, followed by electrophilic cyclization. Journal of Organic Chemistry. 2005, 70 (25), 10292-10296 .
- Adam Morel et. al. Palladium-catalyzed asymmetric Heck arylation of 2,3-dihydrofuran--effect of prolinate salts. Dalton Transactions. 2013, 42 (4), 1215-1222.