Search Thermo Fisher Scientific
Thermo Scientific Chemicals
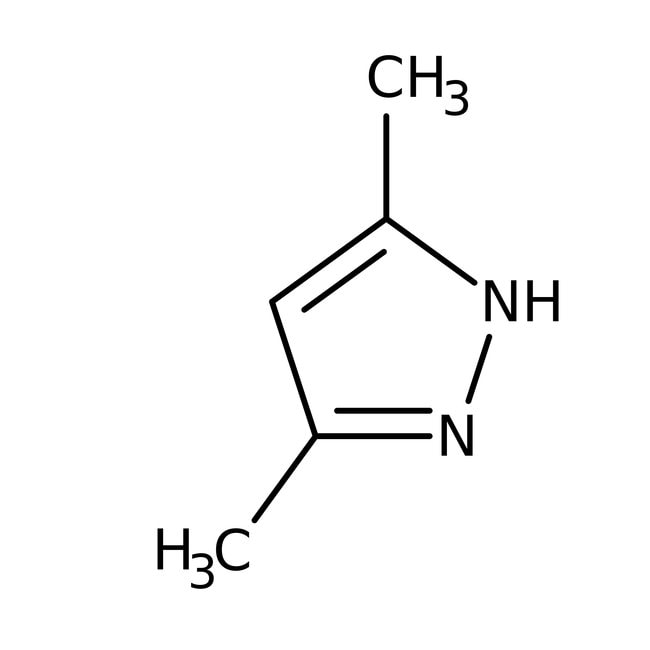
3,5-Dimethyl-1H-pyrazole, 99%, Thermo Scientific Chemicals
Catalog number A10157.0B
also known as A10157-0B
Price (USD)/ Each
336.00
-
Quantity:
1000 g
Price (USD)/ Each
336.00
3,5-Dimethyl-1H-pyrazole, 99%, Thermo Scientific Chemicals
Catalog numberA10157.0B
Price (USD)/ Each
336.00
-
Chemical Identifiers
CAS67-51-6
IUPAC Name3,5-dimethyl-1H-pyrazole
Molecular FormulaC5H8N2
InChI KeySDXAWLJRERMRKF-UHFFFAOYSA-N
SMILESCC1=CC(C)=NN1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pink
Assay (GC)≥98.5%
FormCrystals or powder or crystalline powder
Melting Point (clear melt)104.0-110.0?C
3,5-Dimethyl-1H-pyrazole is combined with Chromium(VI) oxide, is a valuable reagent for oxidation of primary and secondary alcohols to carbonyl compounds. It reacts with malonic esters gives a family of cross-conjugated monomeric betaines. It is a common reagent for the preparation of pyrazolato ligated complexes. It is also used to prepare N-1-substituted derivatives having antibacterial activity.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3,5-Dimethyl-1H-pyrazole is combined with Chromium(VI) oxide, is a valuable reagent for oxidation of primary and secondary alcohols to carbonyl compounds. It reacts with malonic esters gives a family of cross-conjugated monomeric betaines. It is a common reagent for the preparation of pyrazolato ligated complexes. It is also used to prepare N-1-substituted derivatives having antibacterial activity.
Solubility
Soluble in water, methanol 0.1 g/mL.
Notes
Store away from oxidizing agents. Store in a cool, dry condition in well sealed containers.
3,5-Dimethyl-1H-pyrazole is combined with Chromium(VI) oxide, is a valuable reagent for oxidation of primary and secondary alcohols to carbonyl compounds. It reacts with malonic esters gives a family of cross-conjugated monomeric betaines. It is a common reagent for the preparation of pyrazolato ligated complexes. It is also used to prepare N-1-substituted derivatives having antibacterial activity.
Solubility
Soluble in water, methanol 0.1 g/mL.
Notes
Store away from oxidizing agents. Store in a cool, dry condition in well sealed containers.
RUO – Research Use Only
General References:
- Hasnae Bendaha; Lisa Yu; Rachid Touzani; Rachid Souane; Guri Giaever; Corey Nislow; Charles Boone; Sghir El Kadiri; Grant W. Brown; Mohammed Bellaoui. New azole antifungal agents with novel modes of action: Synthesis and biological studies of new tridentate ligands based on pyrazole and triazole. European Journal of Medicinal Chemistry. 2011, 46 (9), 4117-4124.
- F Castro González; A Martínez Garza. Comparative study of analgesics. Practica odontologica. 1986, 7 (2), 30-34.
- The combination with Chromium(VI) oxide, 12522 is a valuable reagent for oxidation of primary and secondary alcohols to carbonyl compounds in very high yields: Tetrahedron Lett., 4499 (1973). With Pyridinium chlorochromate, A11752, allylic alcohols are oxidized selectively in the presence of secondary alcohols: J. Org. Chem., 48, 4766 (1983).
- Reaction of pyrazoles with malonic esters gives a family of cross-conjugated monomeric betaines: Synthesis, 629 (1989):