Search Thermo Fisher Scientific
Thermo Scientific Chemicals
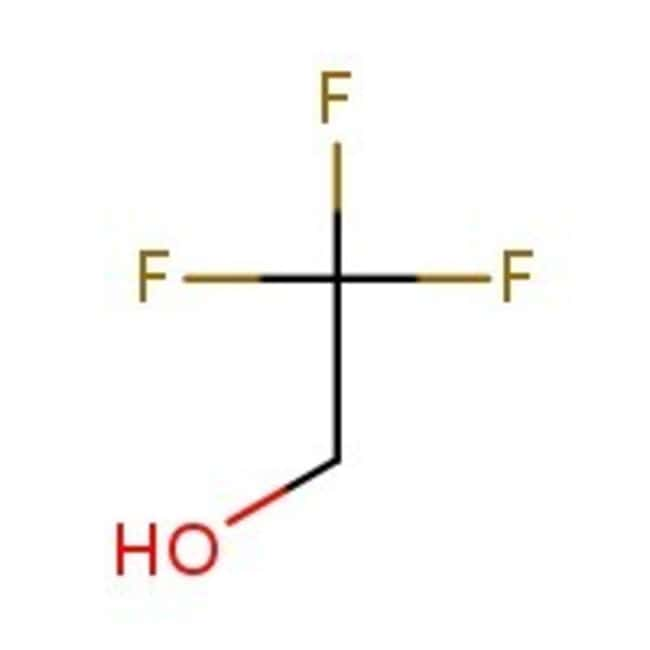
2,2,2-Trifluoroethanol, 99+%, Thermo Scientific Chemicals
Catalog number A10788.18
also known as A10788-18
Price (USD)/ Each
30.65
Online exclusive
33.90 Save 3.25 (10%)
-
Quantity:
50 g
Price (USD)/ Each
30.65
Online exclusive
33.90 Save 3.25 (10%)
2,2,2-Trifluoroethanol, 99+%, Thermo Scientific Chemicals
Catalog numberA10788.18
Price (USD)/ Each
30.65
Online exclusive
33.90 Save 3.25 (10%)
-
Chemical Identifiers
CAS75-89-8
IUPAC Name2,2,2-trifluoroethan-1-ol
Molecular FormulaC2H3F3O
InChI KeyRHQDFWAXVIIEBN-UHFFFAOYSA-N
SMILESOCC(F)(F)F
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
FormLiquid
Assay (GC)≥99.0% (area%, UK sourced material)
Assay from Supplier's CofA≥99.0% (wt% GC, US sourced material)
Identification (FTIR)Conforms
Trifluoroethanol serves as a solvent and a raw material in organic chemistry and biology. TFE is a solvent of choice for hydrogen peroxide-mediated oxidations of sulfides. Trifluoroethanol acts as a protein denaturant. It is used in the manufacture of certain pharmaceuticals and drug substances. The drug fluromer, which is 2,2,2-trifluoro-1-vinyloxyethane, is the vinyl ether of trifluorethanol. It is an effective solvent for peptides and proteins, and used for NMR-based protein folding studies, and in the manufacture of nylon. As a source of the trifluoromethyl group, it is employed in several organic reactions, for example in Still-Gennari modification of Horner-Wadsworth-Emmons reaction (HWE) reaction.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Trifluoroethanol serves as a solvent and a raw material in organic chemistry and biology. TFE is a solvent of choice for hydrogen peroxide-mediated oxidations of sulfides. Trifluoroethanol acts as a protein denaturant. It is used in the manufacture of certain pharmaceuticals and drug substances. The drug fluromer, which is 2,2,2-trifluoro-1-vinyloxyethane, is the vinyl ether of trifluorethanol. It is an effective solvent for peptides and proteins, and used for NMR-based protein folding studies, and in the manufacture of nylon. As a source of the trifluoromethyl group, it is employed in several organic reactions, for example in Still-Gennari modification of Horner-Wadsworth-Emmons reaction (HWE) reaction.
Solubility
Miscible with water, ethers, ketones, alcohols and chloroform.
Notes
Moisture sensitive.
Trifluoroethanol serves as a solvent and a raw material in organic chemistry and biology. TFE is a solvent of choice for hydrogen peroxide-mediated oxidations of sulfides. Trifluoroethanol acts as a protein denaturant. It is used in the manufacture of certain pharmaceuticals and drug substances. The drug fluromer, which is 2,2,2-trifluoro-1-vinyloxyethane, is the vinyl ether of trifluorethanol. It is an effective solvent for peptides and proteins, and used for NMR-based protein folding studies, and in the manufacture of nylon. As a source of the trifluoromethyl group, it is employed in several organic reactions, for example in Still-Gennari modification of Horner-Wadsworth-Emmons reaction (HWE) reaction.
Solubility
Miscible with water, ethers, ketones, alcohols and chloroform.
Notes
Moisture sensitive.
RUO – Research Use Only
General References:
- Useful ionizing solvent, see: Tetrahedron Lett., 2335 (1974), and references therein. Good solvent for oligopeptides with potential as a cosolvent along with proton acceptors such as DMF for peptide coupling reactions: Tetrahedron Lett., 33, 7007 (1992). Compare 1,1,1,3,3,3-Hexafluoro-2-propanol, A12747.
- Effective solvent for uncatalyzed epoxidations of alkenes with hydrogen peroxide (caution! 60%): Synlett, 248 (2001). For a review of fluorinated alcohols as solvents for selective and clean reactions, see: Synlett, 18 (2004).
- Trifluoroethyl esters have also found use as active esters in peptide coupling; see, for example: J. Chem. Soc., Perkin 1, 2867 (1996).
- Reacts with triphenylphosphine dibromide to give the bis(trifluoroethoxy)phosphorane, which converts alcohols to trifluoroethyl ethers, carboxylic acids to trifluoroethyl esters and aldehydes to bis(trifluoroethyl) acetals: J. Org. Chem., 45, 5052 (1980).
- The stereodirecting effect of the MEM group (see 2-Methoxyethoxymethyl chloride, L01050) has been exploited by Percy et al in the formation of a new fluorine-containing acyl anion equivalent: Tetrahedron, 51, 9201 (1995):
- Claisen or Wittig rearrangements of the derived difluoroallyl alcohols can be used in routes to molecules containing a CF2 group: Tetrahedron, 51, 11327 (1995); J. Org. Chem., 61, 166 (1996), or to monofluorinated vinylic compounds: Tetrahedron Lett., 37, 5183 (1996).
- Bava, Y. B.; Martínez, Y. B.; Betancourt, A. M.; Erben, M. F.; Filho, R. L. C.; Védova, C. O. D.; Romano, R. M. Ionic Fragmentation Mechanisms of 2, 2, 2-Trifluoroethanol Following Excitation with Synchrotron Radiation. ChemPhysChem 2015, 16 (2), 322-330.
- Scodeller, I.; Salvini, A.; Manca, G.; Ienco, A.; Luconi, L.; Oberhauser, W. 2,2,2-Trifluoroethanol-assisted imine hydrogenation by a Ru-monohydride. Inorg. Chim. Acta 2015, 431, 242-247.