Search Thermo Fisher Scientific
Thermo Scientific Chemicals
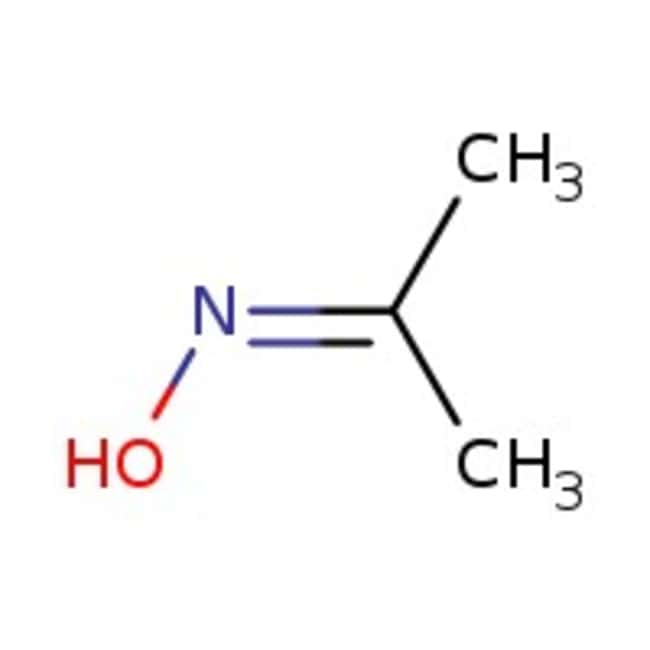
Acetone oxime, 98%, Thermo Scientific Chemicals
Catalog number A10802.30
also known as A10802-30
Price (USD)/ Each
69.70
-
Quantity:
250 g
Price (USD)/ Each
69.70
Acetone oxime, 98%, Thermo Scientific Chemicals
Catalog numberA10802.30
Price (USD)/ Each
69.70
-
Chemical Identifiers
CAS127-06-0
IUPAC NameN-(propan-2-ylidene)hydroxylamine
Molecular FormulaC3H7NO
InChI KeyPXAJQJMDEXJWFB-UHFFFAOYSA-N
SMILESCC(C)=NO
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream
Melting Point (clear melt)57.5-63.5?C
FormCrystals or powder or crystalline powder or fused solid
Assay (GC)≥97.5%
It is an intermediate used in organic synthesis and in agriculture. It is also an important raw material.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is an intermediate used in organic synthesis and in agriculture. It is also an important raw material.
Solubility
Soluble in water (330 g/L at 20°C).
Notes
Store away from oxidizing agents. Protect against electrostatic charges.
It is an intermediate used in organic synthesis and in agriculture. It is also an important raw material.
Solubility
Soluble in water (330 g/L at 20°C).
Notes
Store away from oxidizing agents. Protect against electrostatic charges.
RUO – Research Use Only
General References:
- Jianjun Wu.; Sarah C. Larsen. Solid-State Nuclear Magnetic Resonance Study of Acetone Oxime Adsorbed on CuZSM-5 and on HZSM-5. Journal of Catalysis. 1999, 182, (1), 244-256.
- Karl Sohlberg.; Scott P. Leary.; Noel L. Owen.; Boris A. Trofimov. The infrared spectrum and conformation of acetone oxime vinyl ether. Vibrational Spectroscopy. 1997, 13, (2), 227-234.
- Aldehydes and ketones can be converted to their oximes in high yield by an exchange process involving heating with acetone oxime in acetic acid at 110°: J. Prakt. Chem., 331, 870 (1989).
- N-Protected form of hydroxylamine. For O-alkylation with sodium bromoacetate, and acid hydrolysis to O-carboxymethylhydroxylamine hydrochloride, see: Org. Synth. Coll., 3, 172 (1955).
- For use of this and other simple oximes in the formation, by DCC-coupling, of active esters of N-protected oligopeptides, which react with C-protected amino acids to give peptides in high yield, see: Chem. Pharm. Bull., 17, 2937 (1969). See Appendix 6.