Search Thermo Fisher Scientific
Thermo Scientific Chemicals
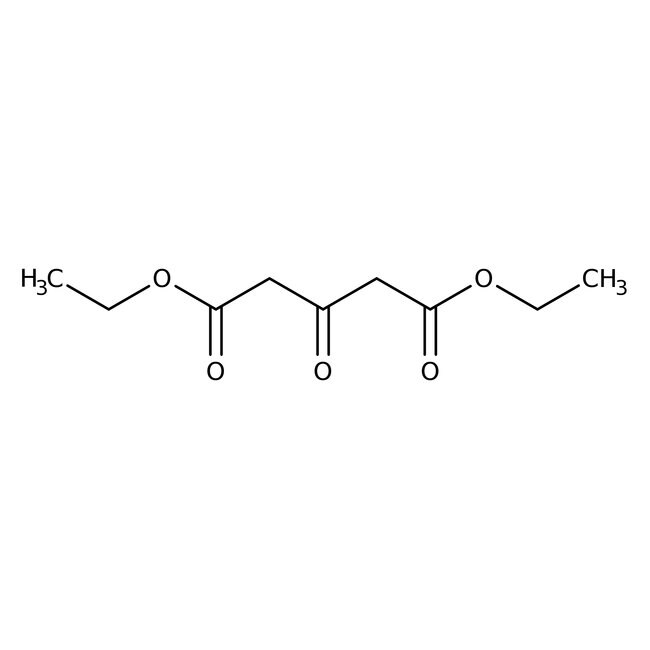
Diethyl acetone-1,3-dicarboxylate, 96%, Thermo Scientific Chemicals
Catalog number A10933.30
also known as A10933-30
Price (USD)/ Each
182.00
-
Quantity:
250 g
Price (USD)/ Each
182.00
Diethyl acetone-1,3-dicarboxylate, 96%, Thermo Scientific Chemicals
Catalog numberA10933.30
Price (USD)/ Each
182.00
-
Chemical Identifiers
CAS105-50-0
IUPAC Name1,5-diethyl 3-oxopentanedioate
Molecular FormulaC9H14O5
InChI KeyZSANYRMTSBBUCA-UHFFFAOYSA-N
SMILESCCOC(=O)CC(=O)CC(=O)OCC
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
FormLiquid
Refractive Index1.4375-1.4435 @ 20?C
Assay (GC)≥95.0%
Identification (FTIR)Conforms
Synthesis of 1-substituted 4-ethoxycarbonyl-5- (ethoxycarbonylmethyl) pyrazoles Diethyl acetone-1, 3-dicarboxylate reacts with N, N-dimethylforma- mide dimethyl acetal (DMFDMA) in ethanol at room temperature. Unexpected product dichotomy is produced in the Biginelli-like condensation of 2-hydroxybenzaldehyde with urea or thiourea and dimethyl or diethyl acetone-1,3-dicarboxylate, respectively, as active methylene components. Ethyl 2-amino-4-(2-ethoxy-2-oxoethyl)thiazole-5-carboxylate (2a), prepared from diethylacetone-1,3-dicarboxylate, sulfuryl chloride and thiourea. Synthesis of diethyl 2,2-diethyl-3,5-dioxopimelate by the reaction of ethyl 3-chloro-3-oxo-2,2-dimethylpropionate with diethyl acetone-1,3-dicarboxylate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Synthesis of 1-substituted 4-ethoxycarbonyl-5- (ethoxycarbonylmethyl) pyrazoles Diethyl acetone-1, 3-dicarboxylate reacts with N, N-dimethylforma- mide dimethyl acetal (DMFDMA) in ethanol at room temperature. Unexpected product dichotomy is produced in the Biginelli-like condensation of 2-hydroxybenzaldehyde with urea or thiourea and dimethyl or diethyl acetone-1,3-dicarboxylate, respectively, as active methylene components. Ethyl 2-amino-4-(2-ethoxy-2-oxoethyl)thiazole-5-carboxylate (2a), prepared from diethylacetone-1,3-dicarboxylate, sulfuryl chloride and thiourea. Synthesis of diethyl 2,2-diethyl-3,5-dioxopimelate by the reaction of ethyl 3-chloro-3-oxo-2,2-dimethylpropionate with diethyl acetone-1,3-dicarboxylate.
Solubility
Insoluble in water and soluble in alcohol, ester, benzene.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
Synthesis of 1-substituted 4-ethoxycarbonyl-5- (ethoxycarbonylmethyl) pyrazoles Diethyl acetone-1, 3-dicarboxylate reacts with N, N-dimethylforma- mide dimethyl acetal (DMFDMA) in ethanol at room temperature. Unexpected product dichotomy is produced in the Biginelli-like condensation of 2-hydroxybenzaldehyde with urea or thiourea and dimethyl or diethyl acetone-1,3-dicarboxylate, respectively, as active methylene components. Ethyl 2-amino-4-(2-ethoxy-2-oxoethyl)thiazole-5-carboxylate (2a), prepared from diethylacetone-1,3-dicarboxylate, sulfuryl chloride and thiourea. Synthesis of diethyl 2,2-diethyl-3,5-dioxopimelate by the reaction of ethyl 3-chloro-3-oxo-2,2-dimethylpropionate with diethyl acetone-1,3-dicarboxylate.
Solubility
Insoluble in water and soluble in alcohol, ester, benzene.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
General References:
- T. A. Bryson,; T. M. Dolak. Dimethyl 2,3-Pentadienedioate. Organic Syntheses.
- Stefanie Reima,; Van Thi Hong Nguyen,; Uwe Albrechta,; Peter Langer. Synthesis of 3,5-dioxoalkanoates, 3,5-dioxopimelates and 2,4-dioxoadipates by acylation of 1,3-bis-silyl enol ethers. Tetrahedron Letters. 2005, 46 (48),8423-8425.
- Valuable synthetic building block, compare Dimethyl acetone-1,3-dicarboxyl ate, A14969. Used in synthesis of isoquinolin-2-ones: Heterocycles, 33, 515 (1992).