Search Thermo Fisher Scientific
Thermo Scientific Chemicals
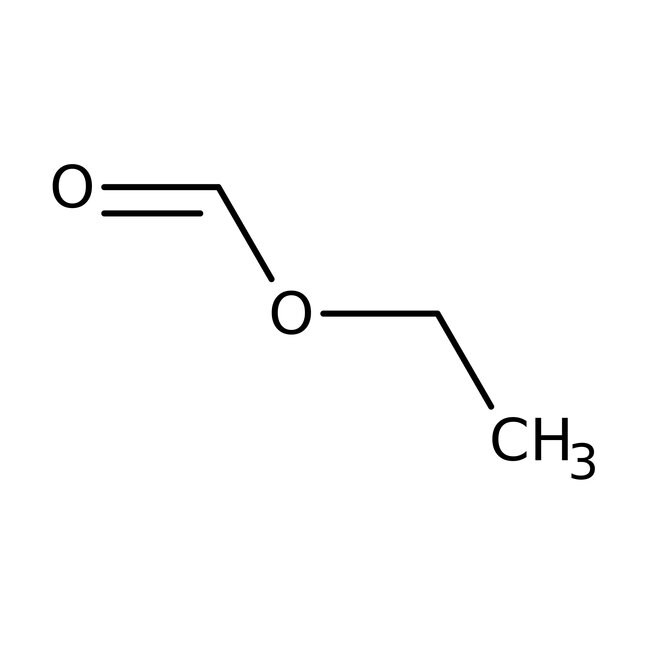
Ethyl formate, 97%, Thermo Scientific Chemicals
Catalog number A11113.AP
also known as A11113-AP
Price (USD)/ Each
42.65
Online exclusive
47.70 Save 5.05 (11%)
-
Quantity:
500 mL
Price (USD)/ Each
42.65
Online exclusive
47.70 Save 5.05 (11%)
Ethyl formate, 97%, Thermo Scientific Chemicals
Catalog numberA11113.AP
Price (USD)/ Each
42.65
Online exclusive
47.70 Save 5.05 (11%)
-
Chemical Identifiers
CAS109-94-4
IUPAC Nameethyl formate
Molecular FormulaC3H6O2
InChI KeyWBJINCZRORDGAQ-UHFFFAOYSA-N
SMILESCCOC=O
View more
Specifications Specification Sheet
Specification Sheet
CommentSpecification differs for U.S. and non-U.S. material where indicated
Identification (FTIR)Conforms (non-U.S. specification)
Refractive Index1.3580-1.3630 @ 20°C
Appearance (Color)Clear colorless to pale yellow
FormLiquid
View more
Ethyl formate is used as a solvent for cellulose nitrate, cellulose acetate, greases and oils. It is also used as a flavor for lemonade and essences. It is involved in the fumigation of dried fruits, as an alternative to methyl bromide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl formate is used as a solvent for cellulose nitrate, cellulose acetate, greases and oils. It is also used as a flavor for lemonade and essences. It is involved in the fumigation of dried fruits, as an alternative to methyl bromide.
Solubility
Miscible with water, benzene, alcohol, ether, acetone and most organic solvents.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with bases and oxidizing agents.
Ethyl formate is used as a solvent for cellulose nitrate, cellulose acetate, greases and oils. It is also used as a flavor for lemonade and essences. It is involved in the fumigation of dried fruits, as an alternative to methyl bromide.
Solubility
Miscible with water, benzene, alcohol, ether, acetone and most organic solvents.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with bases and oxidizing agents.
RUO – Research Use Only
General References:
- Many illustrations of use in formylation of carbanions are given in Organic Syntheses: For more recent examples, see: Org. Synth. Coll., 6, 590, Note 1 (1988); Org. Synth. Coll., 9, 180 (Note 4), 239, 548 (1998).
- ɑ-Formylation of the dilithio derivatives of carboxylic acids, followed by facile decarboxylation, provides an indirect conversion to the corresponding aldehydes: Tetrahedron Lett., 699 (1970); 603 (1974).
- Amines readily give N-formyl derivatives, more easily cleaved by hydrolysis than N-acetyl: Can. J. Chem., 60, 3055 (1982); for an example (L-valinol), see: Org. Synth. Coll., 8, 204 (1993).
- Janssen, L. M.; van Oosten, R.; Paul, C. E.; Arends, I. W.; Hollmann, F. Lipase-catalyzed transesterification of ethyl formate to octyl formate. J. Mol. Catal. B: Enzym. 2014, 105, 7-10.
- Balaganesh, M.; Dash, M. R.; Rajakumar, B. Experimental and Computational Investigation on the Gas Phase Reaction of Ethyl Formate with Cl Atoms. J. Phys. Chem. A 2014, 118 (28), 5272-5278