Search Thermo Fisher Scientific
Thermo Scientific Chemicals
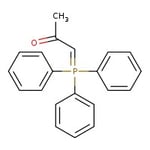
(Acetylmethylene)triphenylphosphorane, 99%, Thermo Scientific Chemicals
Catalog number: A11410.30
250 g, Each


Thermo Scientific Chemicals
(Acetylmethylene)triphenylphosphorane, 99%, Thermo Scientific Chemicals
Catalog number: A11410.30
250 g, Each
Quantity
Catalog number: A11410.30
also known as A11410-30
Price (USD)
Special offer:
671.65 711.65 Online priceSave 40.00 (5%)
Ends: 15-Oct-2024
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
1439-36-7
IUPAC Name
1-(triphenyl-λ⁵-phosphanylidene)propan-2-one
Molecular Formula
C21H19OP
InChI Key
KAANTNXREIRLCT-UHFFFAOYSA-N
SMILES
CC(=O)C=P(C1=CC=CC=C1)(C1=CC=CC=C1)C1=CC=CC=C1
Specifications
Appearance (Color)
White to cream
Form
Crystals or powder or crystalline powder
Assay (HPLC)
≥98.5%
Water Content (Karl Fischer Titration)
≤0.5%
Description
(Acetylmethylene)triphenylphosphorane is used as a Wittig reagent in synthetic chemistry, especially for the synthesis of functionalized pyrrolidines and cyclobutanones. It plays as a vital role in asymmetric allylboration for enantioselective synthesis of (+)-awajanomycin.It is also employed as a reactant in the preparation of 1,2-dioxanes with antitrypanosomal activity. Further, it is used in the preparation of amphibian pyrrolizidine alkaloids through allylic aminations and silicon-containing acyclic dienone musk odorants. In addition to this, it is involved in Domino Suzuki/Heck coupling reactions to prepare fluorenylidenes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(Acetylmethylene)triphenylphosphorane is used as a Wittig reagent in synthetic chemistry, especially for the synthesis of functionalized pyrrolidines and cyclobutanones. It plays as a vital role in asymmetric allylboration for enantioselective synthesis of (+)-awajanomycin.It is also employed as a reactant in the preparation of 1,2-dioxanes with antitrypanosomal activity. Further, it is used in the preparation of amphibian pyrrolizidine alkaloids through allylic aminations and silicon-containing acyclic dienone musk odorants. In addition to this, it is involved in Domino Suzuki/Heck coupling reactions to prepare fluorenylidenes.
Solubility
Soluble in chloroform. Slightly soluble in methanol.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
(Acetylmethylene)triphenylphosphorane is used as a Wittig reagent in synthetic chemistry, especially for the synthesis of functionalized pyrrolidines and cyclobutanones. It plays as a vital role in asymmetric allylboration for enantioselective synthesis of (+)-awajanomycin.It is also employed as a reactant in the preparation of 1,2-dioxanes with antitrypanosomal activity. Further, it is used in the preparation of amphibian pyrrolizidine alkaloids through allylic aminations and silicon-containing acyclic dienone musk odorants. In addition to this, it is involved in Domino Suzuki/Heck coupling reactions to prepare fluorenylidenes.
Solubility
Soluble in chloroform. Slightly soluble in methanol.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text