Search Thermo Fisher Scientific
Thermo Scientific Chemicals
1H-Indazole, 99%, Thermo Scientific Chemicals
Catalog number A11665.03
also known as A11665-03
Price (USD)/ Each
38.50
-
Quantity:
1 g
Price (USD)/ Each
38.50
1H-Indazole, 99%, Thermo Scientific Chemicals
Catalog numberA11665.03
Price (USD)/ Each
38.50
-
Chemical Identifiers
CAS271-44-3
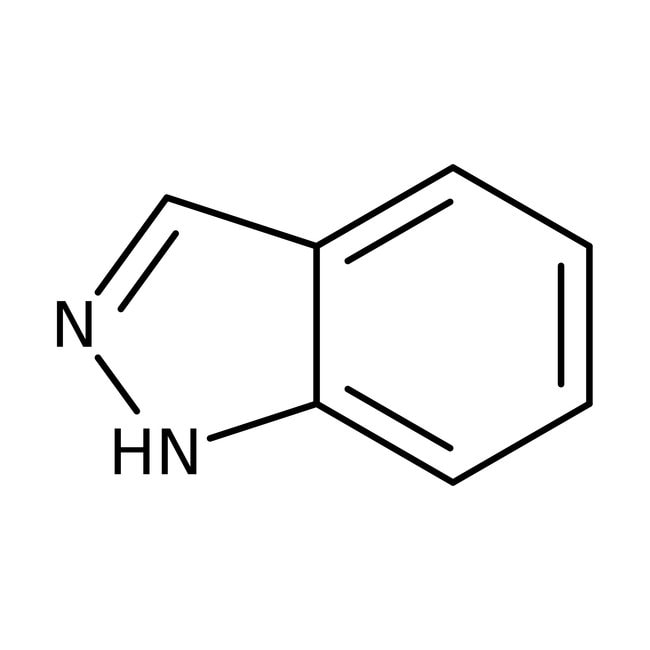
IUPAC Name1H-indazole
Molecular FormulaC7H6N2
InChI KeyBAXOFTOLAUCFNW-UHFFFAOYSA-N
SMILESN1N=CC2=CC=CC=C12
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥98.5%
FormCrystals or powder or crystalline powder
Melting Point (clear melt)144.0-150.0?C
Appearance (Color)White to cream
Identification (FTIR)Conforms
1H-Indazole is used in organic synthesis, it can react with butyryl chloride and 1-butyryl-1H-indazole. It is also used in synthesis of several small molecule inhibitors as potential cancer therapeutics.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1H-Indazole is used in organic synthesis, it can react with butyryl chloride and 1-butyryl-1H-indazole. It is also used in synthesis of several small molecule inhibitors as potential cancer therapeutics.
Solubility
Soluble in hot water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
1H-Indazole is used in organic synthesis, it can react with butyryl chloride and 1-butyryl-1H-indazole. It is also used in synthesis of several small molecule inhibitors as potential cancer therapeutics.
Solubility
Soluble in hot water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- Barbara Pergolese.; Adriano Bigotto. Surface enhanced Raman spectroscopic studies of 1H-indazole on silver sols. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2001, 57, (6), 1191-1197.
- Betty Cottyn.; Francine Acher.; Booma Ramassamy.; Luke Alvey.; Michel Lepoivre.; Yves Frapart.; Dennis Stuehr.; Daniel Mansuy.; Jean-Luc Boucher.; Dominique Vichar. Inhibitory effects of a series of 7-substituted-indazoles toward nitric oxide syntheses: Particular potency of 1H-indazole-7-carbonitrile. Bioorganic & Medicinal Chemistry. 2008, 16, (11), 5962-5973.