Search Thermo Fisher Scientific
Thermo Scientific Chemicals
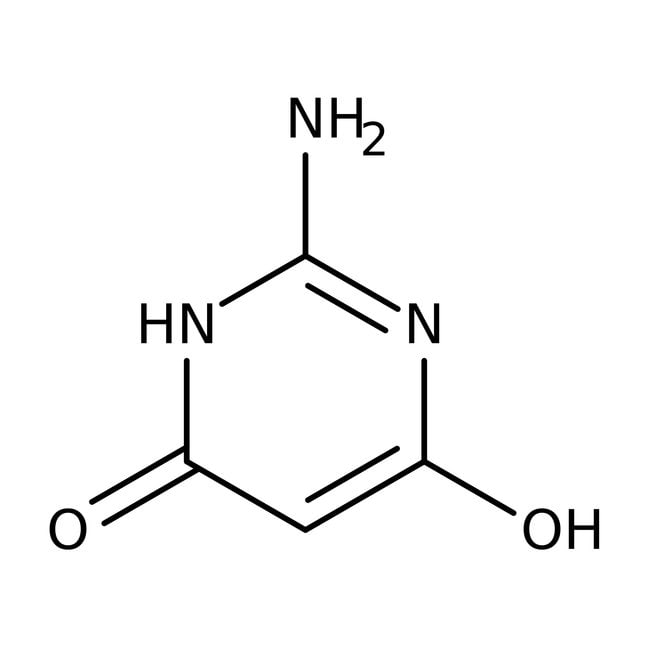
2-Amino-4,6-dihydroxypyrimidine, 98%, Thermo Scientific Chemicals
Catalog number A11813.22
also known as A11813-22
Price (USD)/ Each
156.00
-
Quantity:
100 g
Price (USD)/ Each
156.00
2-Amino-4,6-dihydroxypyrimidine, 98%, Thermo Scientific Chemicals
Catalog numberA11813.22
Price (USD)/ Each
156.00
-
Chemical Identifiers
CAS56-09-7
IUPAC Name2-amino-6-hydroxy-3,4-dihydropyrimidin-4-one
Molecular FormulaC4H5N3O2
InChI KeyAUFJTVGCSJNQIF-UHFFFAOYSA-N
SMILESNC1=NC(O)=CC(=O)N1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream or pale yellow to pale pink
FormPowder
Elemental AnalysisC: 37.80 ? 1.0%
Elemental AnalysisH: 3.97 ? 0.2%
Elemental AnalysisN: 33.06 ? 0.8%
View more
2-Amino-4,6-dihydroxypyrimidine acts as an intermediate in the production of antimicrobial guanylsulfonamides. It also acts as an intermediate in pharmaceuticals and agrochemicals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Amino-4,6-dihydroxypyrimidine acts as an intermediate in the production of antimicrobial guanylsulfonamides. It also acts as an intermediate in pharmaceuticals and agrochemicals.
Solubility
Soluble in Aqueous Alkali.
Notes
Store away from oxidizing agents. Store at -20°C. Store in a cool, dry, well-ventilated area.
2-Amino-4,6-dihydroxypyrimidine acts as an intermediate in the production of antimicrobial guanylsulfonamides. It also acts as an intermediate in pharmaceuticals and agrochemicals.
Solubility
Soluble in Aqueous Alkali.
Notes
Store away from oxidizing agents. Store at -20°C. Store in a cool, dry, well-ventilated area.
RUO – Research Use Only
General References:
- V. Krishnakumar and N. Prabavathi. DFT simulations and vibrational analysis of FTIR and FT-Raman spectra of 2-amino-4,6-dihydroxypyrimidine.Journal of Raman Spectroscopy. 2008, 39 679-684.
- A. Bacher and F. Lingens. Biosynthesis of Riboflavin Formation of 6-hydroxy-2,4,5-triaminopyrimidine in rib7 mutants of saccharomyces cerevisiae.Journal of Biological Chemistry. 1971, 246 7018-7022..
- Can be used catalytically as a ligand in combination with palladium acetate for the efficient Suzuki-Miyaura cross-coupling reaction of arylboronic acids with aryl halides: Synlett, 1897 (2005).