Search Thermo Fisher Scientific
Thermo Scientific Chemicals
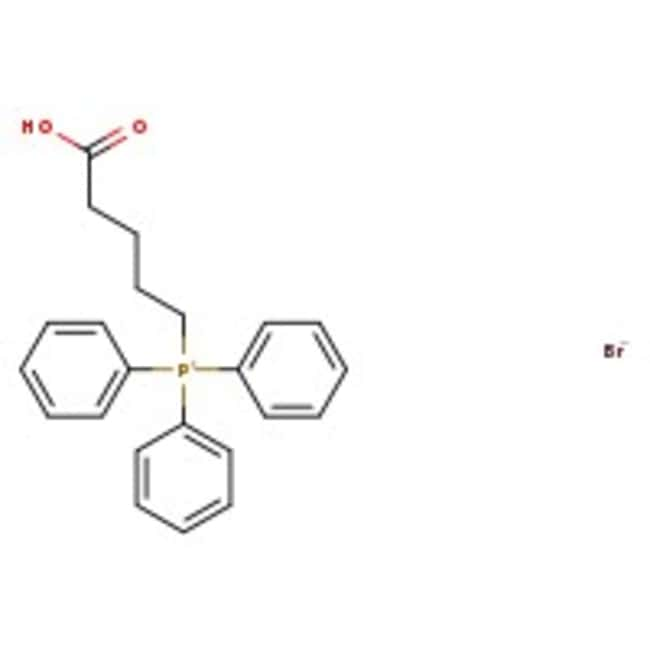
(4-Carboxybutyl)triphenylphosphonium bromide, 98%, Thermo Scientific Chemicals
Catalog number A12023.36
also known as A12023-36
Price (USD)/ Each
489.65
Online exclusive
544.00 Save 54.35 (10%)
-
Quantity:
500 g
Price (USD)/ Each
489.65
Online exclusive
544.00 Save 54.35 (10%)
(4-Carboxybutyl)triphenylphosphonium bromide, 98%, Thermo Scientific Chemicals
Catalog numberA12023.36
Price (USD)/ Each
489.65
Online exclusive
544.00 Save 54.35 (10%)
-
Chemical Identifiers
CAS17814-85-6
IUPAC Name(4-carboxybutyl)triphenylphosphanium bromide
Molecular FormulaC23H24BrO2P
InChI KeyMLOSJPZSZWUDSK-UHFFFAOYSA-N
SMILES[Br-].OC(=O)CCCC[P+](C1=CC=CC=C1)(C1=CC=CC=C1)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream to pale yellow
Purity≥97.5% (HPLC)
FormSolid
Phosphonium salt is a key intermediate for manufacture of some types of prostaglandins, which are subsequently used as active ingredients of both human and veterinary drugs.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phosphonium salt is a key intermediate for manufacture of some types of prostaglandins, which are subsequently used as active ingredients of both human and veterinary drugs.
Solubility
Soluble in ethanol, methanol and soluble water. Insoluble in toluene and hexane.
Notes
Hygroscopic. Store in dry place. Keep away from oxidizing agents.
Phosphonium salt is a key intermediate for manufacture of some types of prostaglandins, which are subsequently used as active ingredients of both human and veterinary drugs.
Solubility
Soluble in ethanol, methanol and soluble water. Insoluble in toluene and hexane.
Notes
Hygroscopic. Store in dry place. Keep away from oxidizing agents.
RUO – Research Use Only
General References:
- Yoshinori Yamamoto.; Toshiaki Komatsu.; Kazuhiro Maruyama. Diastereofacial selectivity in the reaction of allylic organometallic compounds with imines. Stereoelectronic effect of imine group.J. Org. Chem. 1985, 50 (17),3115-3121.
- Robert Deschenaux.; Martin Schweissguth.; Maria-Teresa Vilches. Switchable Mesomorphic Materials Based on the Ferrocene-Ferrocenium Redox System: Electron-Transfer-Generated Columnar Liquid-Crystalline Phases. Organometallics. 1999, 18 (26),5553-5559.
- Use of LiHMDS as base in the Wittig reaction with aromatic aldehydes gives a much higher proportion of the trans-alkene than alternative bases such as dimsyl sodium or KO-t-Bu: Tetrahedron Lett., 22, 4185 (1981). See Appendix 1.