Search Thermo Fisher Scientific
Thermo Scientific Chemicals
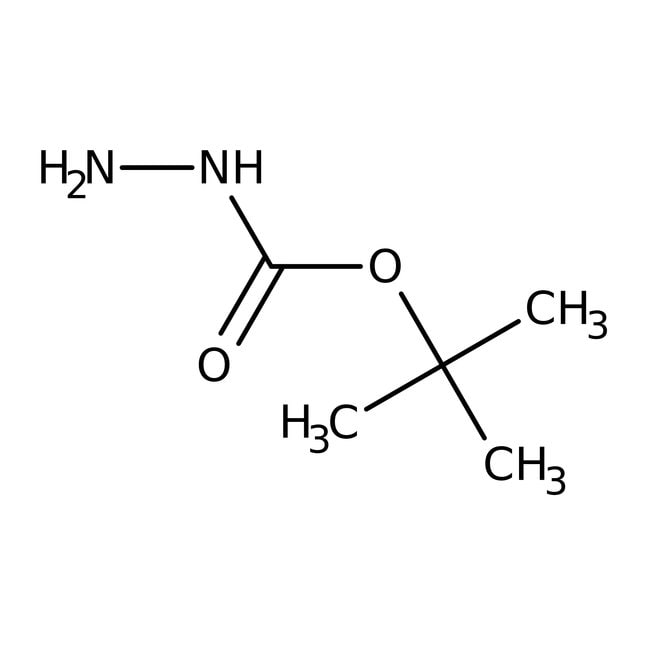
tert-Butyl carbazate, 98+%, Thermo Scientific Chemicals
Catalog number A12383.14
also known as A12383-14
Price (USD)/ Each
66.65
Online exclusive
74.20 Save 7.55 (10%)
-
Quantity:
25 g
Price (USD)/ Each
66.65
Online exclusive
74.20 Save 7.55 (10%)
tert-Butyl carbazate, 98+%, Thermo Scientific Chemicals
Catalog numberA12383.14
Price (USD)/ Each
66.65
Online exclusive
74.20 Save 7.55 (10%)
-
Chemical Identifiers
CAS870-46-2
IUPAC Name(tert-butoxy)carbohydrazide
Molecular FormulaC5H12N2O2
InChI KeyDKACXUFSLUYRFU-UHFFFAOYSA-N
SMILESCC(C)(C)OC(=O)NN
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Assay (GC)>98.0%
Solution Test5% w/v in ethanol: clear solution
Formcrystals or fused solid
tert-Butyl carbazate is used in a palladium-catalyzed cross-coupling with vinyl halides to prepare N-Boc-N-alkenylhydrazines. It is utilized in solid phase peptide synthesis and in the optical purity determination of alfa-amino aldehyde. It reacts with aldehydes to get hydrazones, which finds application as an intermediate in the synthesis of HIV-1 protease inhibitors. In addition to this, it is used as a starting material for the preparation of BOC-azide, sulfonic and carboxylic hydrazides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
tert-Butyl carbazate is used in a palladium-catalyzed cross-coupling with vinyl halides to prepare N-Boc-N-alkenylhydrazines. It is utilized in solid phase peptide synthesis and in the optical purity determination of alfa-amino aldehyde. It reacts with aldehydes to get hydrazones, which finds application as an intermediate in the synthesis of HIV-1 protease inhibitors. In addition to this, it is used as a starting material for the preparation of BOC-azide, sulfonic and carboxylic hydrazides.
Solubility
Soluble in ethanol and methanol.
Notes
Store in a cool place. Incompatible with strong oxidizing agents and strong bases.
tert-Butyl carbazate is used in a palladium-catalyzed cross-coupling with vinyl halides to prepare N-Boc-N-alkenylhydrazines. It is utilized in solid phase peptide synthesis and in the optical purity determination of alfa-amino aldehyde. It reacts with aldehydes to get hydrazones, which finds application as an intermediate in the synthesis of HIV-1 protease inhibitors. In addition to this, it is used as a starting material for the preparation of BOC-azide, sulfonic and carboxylic hydrazides.
Solubility
Soluble in ethanol and methanol.
Notes
Store in a cool place. Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Protected hydrazine derivative, permitting selective reaction at one N atom, followed by mild acidic cleavage of the Boc group. For preparation of, e.g. N-aminosuccinimide, free from the cyclic hydrazide, see: J. Org. Chem., 37, 2040 (1972). Similarly, reaction with carbonyl or sulfonyl halides, followed by cleavage with HCl, provides a route to monoacyl hydrazides: Synthesis, 244 (1980). It is also commonly used in the synthesis of aza-amino acids; see, for example: Synthesis, 141 (1991) and references therein.
- Monoalkylhydrazines can be prepared via reduction of Boc-hydrazones with borane-THF, and cleavage with HCl: J. Org. Chem., 46, 5413 (1981).
- Precursor of the somewhat unstable tert-butyl azidoformate, useful for introduction of the Boc protecting group, see: Org. Synth. Coll., 5, 157 (1973). Caution! Possible violent decomposition of this azide on heating.
- Karpenko, I. A.; Margathe, J. F.; Rodriguez, T.; Pflimlin, E.; Dupuis, E.; Hibert, M.; Durroux, T.; Bonnet, D. Selective Nonpeptidic Fluorescent Ligands for Oxytocin Receptor: Design, Synthesis, and Application to Time-Resolved FRET Binding Assay. J. Med. Chem. 2015, 58 (5), 2547-2552.
- Kheirabadi, M.; Shi, L.; Bagheri, R.; Kabiri, K.; Hilborn, J.; Ossipov, D. A. In situ forming interpenetrating hydrogels of hyaluronic acid hybridized with iron oxide nanoparticles. Biomater. Sci. 2015, 3 (11), 1466-1474.