Search Thermo Fisher Scientific
Thermo Scientific Chemicals
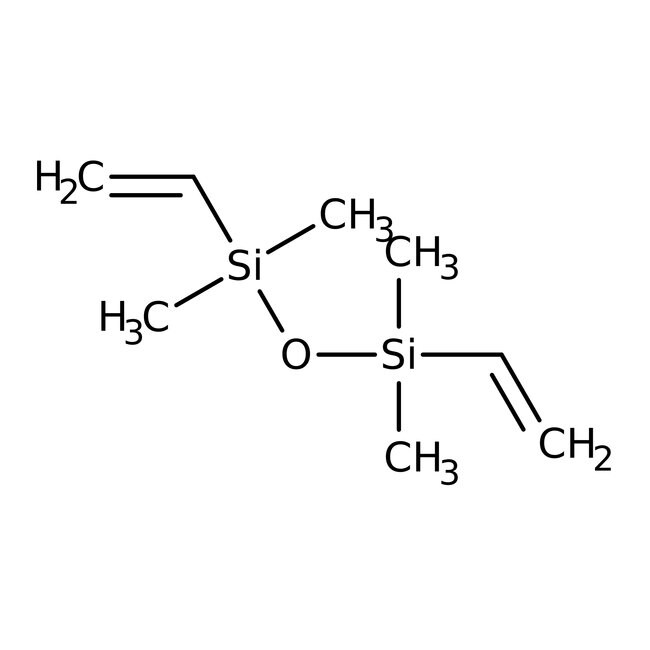
1,3-Divinyltetramethyldisiloxane, 96%, cont. up to 4% 1-vinyl-3-ethyltetramethyldisiloxane, Thermo Scientific Chemicals
Catalog number A12463.14
also known as A12463-14
Price (USD)/ Each
73.40
-
Quantity:
25 g
Price (USD)/ Each
73.40
1,3-Divinyltetramethyldisiloxane, 96%, cont. up to 4% 1-vinyl-3-ethyltetramethyldisiloxane, Thermo Scientific Chemicals
Catalog numberA12463.14
Price (USD)/ Each
73.40
-
Chemical Identifiers
CAS2627-95-4
IUPAC Nameethenyl[(ethenyldimethylsilyl)oxy]dimethylsilane
Molecular FormulaC8H18OSi2
InChI KeyBITPLIXHRASDQB-UHFFFAOYSA-N
SMILESC[Si](C)(O[Si](C)(C)C=C)C=C
View more
Specifications Specification Sheet
Specification Sheet
Identification (FTIR)Conforms
Appearance (Color)Clear colorless
Assay (GC)≥95.0%
Refractive Index1.4080-1.4140 @ 20?C
FormLiquid
1,3-Divinyltetramethyldisiloxane acts as a potential vinyl donor used in cross-coupling reactions. It is also involved in the copolymerization reaction with aromatic ketones.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3-Divinyltetramethyldisiloxane acts as a potential vinyl donor used in cross-coupling reactions. It is also involved in the copolymerization reaction with aromatic ketones.
Solubility
Immiscible with water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents.
1,3-Divinyltetramethyldisiloxane acts as a potential vinyl donor used in cross-coupling reactions. It is also involved in the copolymerization reaction with aromatic ketones.
Solubility
Immiscible with water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Marciniec, B.; Kownacka, A.; Kownacki, I.; Hoffmann, M.; Taylor, R. Hydrosilylation vs. dehydrogenative silylation of styrene catalysed by iron(0) carbonyl complexes with multivinylsilicon ligands - Mechanistic implications. J. Organomet. Chem. 2015, 791, 58-65.
- Dierick, S.; Vercruysse, E.; Berthon-Gelloz, G.; Markó, I. E. User-Friendly Platinum Catalysts for the Highly Stereoselective Hydrosilylation of Alkynes and Alkenes. Chem. Eur. J. 2015, 21 (47), 17073-17078.