Search Thermo Fisher Scientific
Thermo Scientific Chemicals
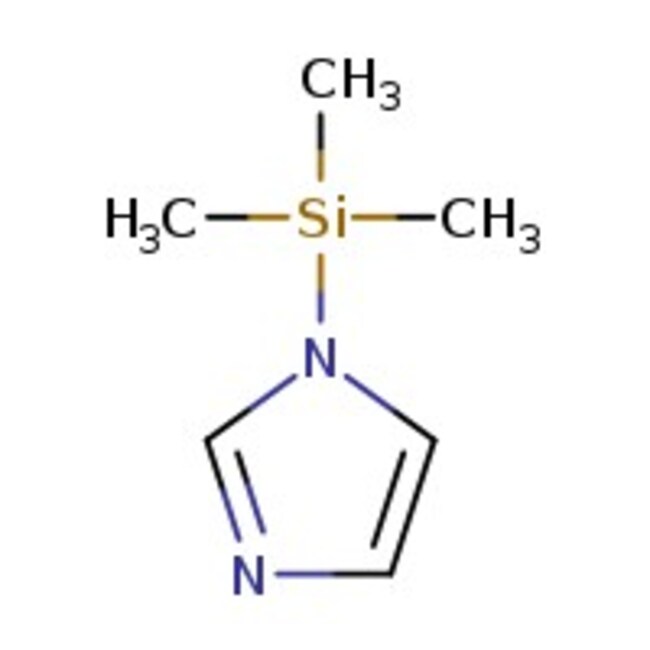
1-(Trimethylsilyl)imidazole, 97%, Thermo Scientific Chemicals
Catalog number A12512.06
also known as A12512-06
Price (USD)/ Each
21.70
-
Quantity:
5 g
Price (USD)/ Each
21.70
1-(Trimethylsilyl)imidazole, 97%, Thermo Scientific Chemicals
Catalog numberA12512.06
Price (USD)/ Each
21.70
-
Chemical Identifiers
CAS18156-74-6
IUPAC Name1-(trimethylsilyl)-1H-imidazole
Molecular FormulaC6H12N2Si
InChI KeyYKFRUJSEPGHZFJ-UHFFFAOYSA-N
SMILESC[Si](C)(C)N1C=CN=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
Assay (GC)≥96.0%
Identification (FTIR)Conforms
Refractive Index1.4740-1.4790 @ 20?C
FormLiquid
1-(Trimethylsilyl)imidazole acts as a silylating agent for alcohols, carbohydrates and 1,3-dicarbonyl compounds. It is a derivatization reagent used for selective silylation of hydroxyl groups and carboxylic acid without affecting the amino group. It employed as an intermediate for the synthesis of imidazole derivatives. It plays an essential role to synthesize polysubstituted chiral spirotetrahydropyrans. It is also used in the quantification of pintol after derivatization by gas chromatography/ mass spectrometry.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-(Trimethylsilyl)imidazole acts as a silylating agent for alcohols, carbohydrates and 1,3-dicarbonyl compounds. It is a derivatization reagent used for selective silylation of hydroxyl groups and carboxylic acid without affecting the amino group. It employed as an intermediate for the synthesis of imidazole derivatives. It plays an essential role to synthesize polysubstituted chiral spirotetrahydropyrans. It is also used in the quantification of pintol after derivatization by gas chromatography/ mass spectrometry.
Solubility
Miscible with most common organic solvents. It decomposes in water.
Notes
Moisture and light sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong acids.
1-(Trimethylsilyl)imidazole acts as a silylating agent for alcohols, carbohydrates and 1,3-dicarbonyl compounds. It is a derivatization reagent used for selective silylation of hydroxyl groups and carboxylic acid without affecting the amino group. It employed as an intermediate for the synthesis of imidazole derivatives. It plays an essential role to synthesize polysubstituted chiral spirotetrahydropyrans. It is also used in the quantification of pintol after derivatization by gas chromatography/ mass spectrometry.
Solubility
Miscible with most common organic solvents. It decomposes in water.
Notes
Moisture and light sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong acids.
WARNING: Reproductive Harm - www.P65Warnings.ca.gov
RUO – Research Use Only
General References:
- Powerful silylating agent (see Appendix 4) for a wide range of functional groups. O-Silylation in the presence of TBAF (0.02 equiv.) occurs under very mild conditions: Tetrahedron Lett., 35, 8409 (1994).
- Acanski, M. M.; Vujic, D. N. Comparing sugar components of cereal and pseudocereal flour by GC-MS analysis. Food Chem. 2014, 145, 743-748.
- Bu, J.; Rhee, H.-K. Silylation of Ti-MCM-41 by trimethylsilyl-imidazole and its effect on the olefin epoxidation with aqueous H2O2. Catal. Lett. 2000, 66 (4), 245-249.
- Jacolot, M.; Jean, M.; Levoin, N.; Weghe, P. The Prins Reaction Using Ketones: Rationalization and Application toward the Synthesis of the Portentol Skeleton. Org. Lett. 2012, 14 (1), 58-61.