Search Thermo Fisher Scientific
Thermo Scientific Chemicals
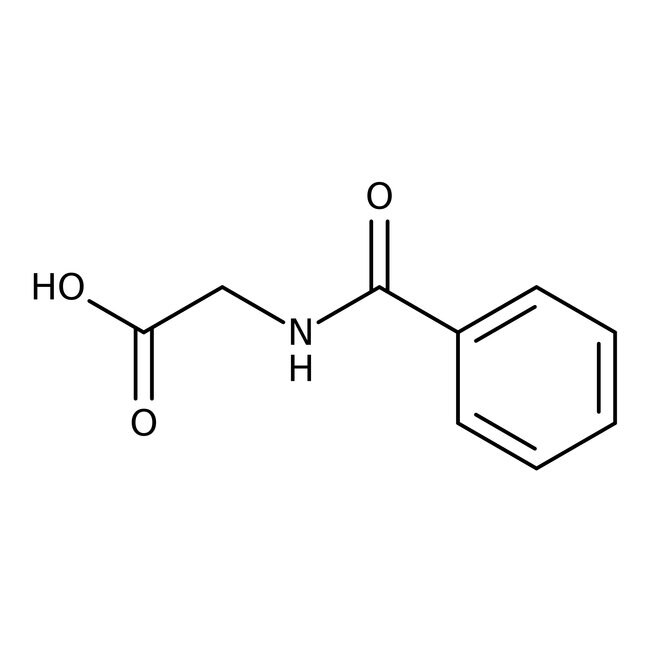
Hippuric acid, 98%, Thermo Scientific Chemicals
Catalog number A12690.36
also known as A12690-36
Price (USD)/ Each
93.50
-
Quantity:
500 g
Price (USD)/ Each
93.50
Hippuric acid, 98%, Thermo Scientific Chemicals
Catalog numberA12690.36
Price (USD)/ Each
93.50
-
Chemical Identifiers
CAS495-69-2
IUPAC Name2-(phenylformamido)acetic acid
Molecular FormulaC9H9NO3
InChI KeyQIAFMBKCNZACKA-UHFFFAOYSA-N
SMILESOC(=O)CNC(=O)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Identification (FTIR)Conforms
Appearance (Color)White
Assay (Aqueous acid-base Titration)≥97.5 to ≤102.5%
Melting Point (clear melt)187.0-194.0?C
FormCrystals or powder or crystalline powder
Hippuric acid is used as a intermediate for the manufacturing medicine and other organic compounds. Hippuric acid can be used to study cell biology, chemical biology, bioactive small molecules, amino acid derivatives, peptide synthesis, chemical synthesis and nutrition. Hippuric acid has been used to inform the metabolism and urinary excretion of procyanidins.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hippuric acid is used as a intermediate for the manufacturing medicine and other organic compounds. Hippuric acid can be used to study cell biology, chemical biology, bioactive small molecules, amino acid derivatives, peptide synthesis, chemical synthesis and nutrition. Hippuric acid has been used to inform the metabolism and urinary excretion of procyanidins.
Solubility
Soluble in water.
Notes
Store in cool dry place in tightly closed container. With good ventilation. Store away from oxidizing agent.
Hippuric acid is used as a intermediate for the manufacturing medicine and other organic compounds. Hippuric acid can be used to study cell biology, chemical biology, bioactive small molecules, amino acid derivatives, peptide synthesis, chemical synthesis and nutrition. Hippuric acid has been used to inform the metabolism and urinary excretion of procyanidins.
Solubility
Soluble in water.
Notes
Store in cool dry place in tightly closed container. With good ventilation. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- A. N. Phippsa; J. Stewart; B. Wright; I. D. Wilson. Effect Of Diet On The Urinary Excretion Of Hippuric Acid And Other Dietary-Derived Aromatics In Rat. A Complex Interaction Between Diet, Gut Microflora And Substrate Specificity. Xenobiotica: The Fate Of Foreign Compounds In Biological Systems. 1998, 28(5), 527-537.
- Quick, Aemand J. Ph.D., M.D.; Cooper, Mary A. B.S. The Synthesis Of Hippuric Acid: A New Test Of Liver Function. American Journal Of The Medical Sciences. 1933, 185(5), 630-635.
- Condensation with aldehydes gives azlactones, which are intermediates in a classical route to amino acids; see, e.g.: Org. Synth. Coll., 2, 489 (1943). An alternative approach involves alkylation of the trianion of hippuric acid, prepared by LDA/TMEDA in THF: Tetrahedron Lett., 2205 (1976).
- For cyclization with acetic anhydride to 2-phenyl-5-oxazolone, see: Org. Synth. Coll., 5, 946 (1973).