Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Tetrabromothiophene, 99%, Thermo Scientific Chemicals
Catalog number A13009.14
also known as A13009-14
Price (USD)/ Each
103.00
-
Quantity:
25 g
Price (USD)/ Each
103.00
Tetrabromothiophene, 99%, Thermo Scientific Chemicals
Catalog numberA13009.14
Price (USD)/ Each
103.00
-
Chemical Identifiers
CAS3958-03-0
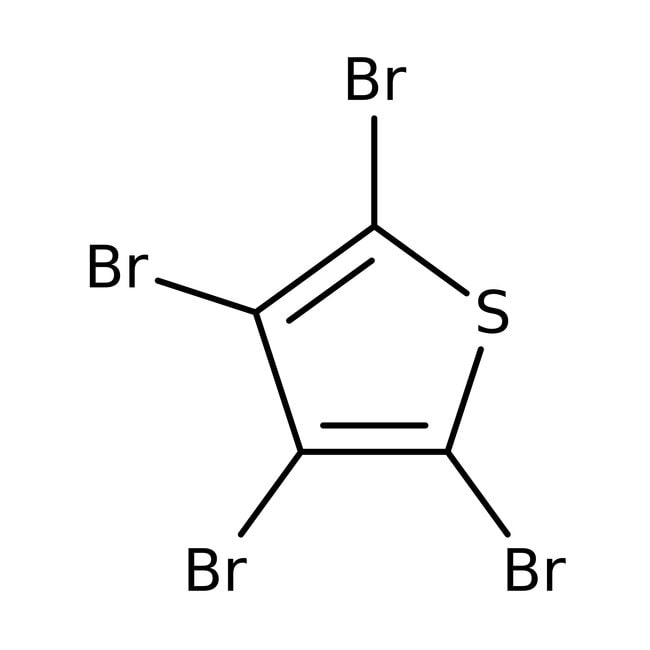
IUPAC Nametetrabromothiophene
Molecular FormulaC4Br4S
InChI KeyAVPWUAFYDNQGNZ-UHFFFAOYSA-N
SMILESBrC1=C(Br)C(Br)=C(Br)S1
View more
Specifications Specification Sheet
Specification Sheet
Melting Point (clear melt)112.0-119.0?C
Appearance (Color)White to pale cream to pale brown
Assay (GC)≥98.5%
FormCrystals or powder or crystalline powder
Tetrabromothiophene, holds wide applications in organic electronics and photonics. It is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetrabromothiophene, holds wide applications in organic electronics and photonics. It is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff.
Solubility
Insoluble in water. Soluble in organic solvents.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong bases, strong oxidizing agents. Stable under normal temperatures and pressures.
Tetrabromothiophene, holds wide applications in organic electronics and photonics. It is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff.
Solubility
Insoluble in water. Soluble in organic solvents.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong bases, strong oxidizing agents. Stable under normal temperatures and pressures.
RUO – Research Use Only
General References:
- TT Dang.; N Rasool.; TT Dang.; H Reinke.; P Langer. Synthesis of tetraarylthiophenes by regioselective Suzuki cross-coupling reactions oftetrabromothiophene. Tetrahedron letters. 200748 (5) , 845-847.
- A Rahimi.; JC Namyslo.; MHH Drafz. Selective Mono-to Perarylations of Tetrabromothiophene by a Cyclobutene-1, 2-diylbisimidazolium Preligand. J. Org. Chem. 201176 (18) , 7316-7325.