Search Thermo Fisher Scientific
Thermo Scientific Chemicals
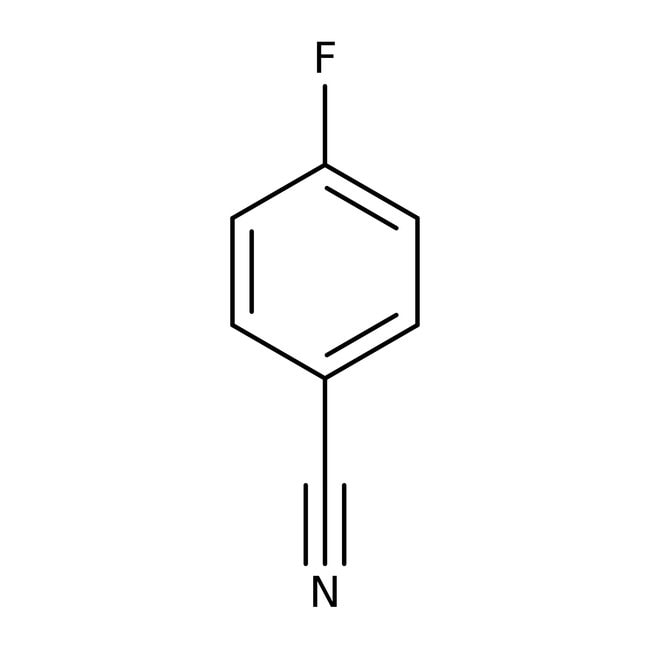
4-Fluorobenzonitrile, 99%, Thermo Scientific Chemicals
Catalog number A13028.06
also known as A13028-06
Price (USD)/ Each
28.90
-
Quantity:
5 g
Price (USD)/ Each
28.90
4-Fluorobenzonitrile, 99%, Thermo Scientific Chemicals
Catalog numberA13028.06
Price (USD)/ Each
28.90
-
Chemical Identifiers
CAS1194-02-1
IUPAC Name4-fluorobenzonitrile
Molecular FormulaC7H4FN
InChI KeyAEKVBBNGWBBYLL-UHFFFAOYSA-N
SMILESFC1=CC=C(C=C1)C#N
View more
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder or fused solid or irregular pieces
Identification (FTIR)Conforms
Appearance (Color)White to cream to yellow
Assay (GC)≥98.5%
4-Fluorobenzonitrile is used as chemical intermediate, solvent for perfumes and pharmaceuticals, stabilizer for chlorinated solvents, HPLC analysis, catalyst and component of transition-metal complex catalysts.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Fluorobenzonitrile is used as chemical intermediate, solvent for perfumes and pharmaceuticals, stabilizer for chlorinated solvents, HPLC analysis, catalyst and component of transition-metal complex catalysts.
Solubility
Insoluble in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
4-Fluorobenzonitrile is used as chemical intermediate, solvent for perfumes and pharmaceuticals, stabilizer for chlorinated solvents, HPLC analysis, catalyst and component of transition-metal complex catalysts.
Solubility
Insoluble in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Eric J.Schelter; David E.Morris; Brian L.Scott; Jaqueline L.Kiplinger. Actinide-mediated coupling of 4-fluorobenzonitrile: synthesis of an eight-membered thorium(IV) tetraazametallacycle. Chemical Communications. 2007, (10),1029-1031
- A.Muthukrishnan; M.V.Sangaranarayanan. Electrochemical reduction of carbon-fluorine bond in 4-fluorobenzonitrile - Mechanistic analysis employing Marcus-Hush quadratic activation-driving force relation. Chemical Physics Letters. 2007, 446, (4-6),297-303
- Displacement of the fluoro-substituent by phenols or thiophenols, to give the corresponding diaryl ethers or thioethers, is promoted by KF on alumina in the presence of 18-crown-6: J. Org. Chem., 58, 3229 (1993).