Search Thermo Fisher Scientific
Thermo Scientific Chemicals
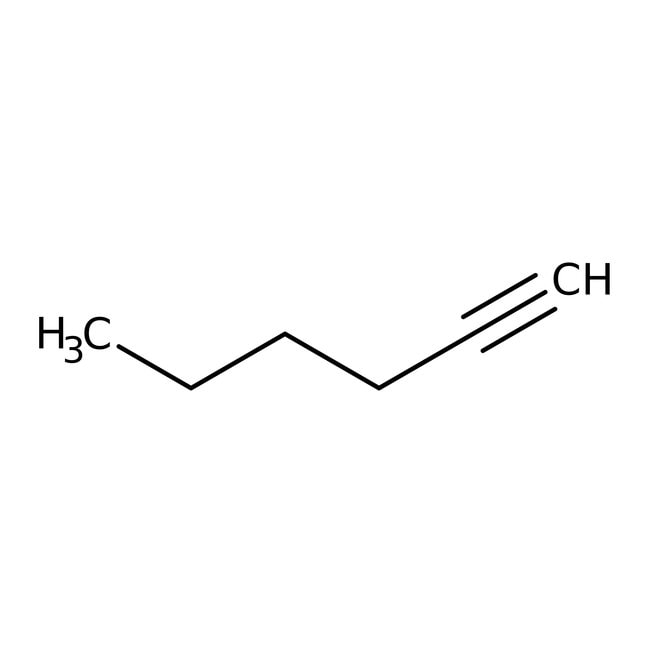
1-Hexyne, 98+%, Thermo Scientific Chemicals
Catalog number A13156.09
also known as A13156-09
Price (USD)/ Each
30.10
-
Quantity:
10 g
Price (USD)/ Each
30.10
1-Hexyne, 98+%, Thermo Scientific Chemicals
Catalog numberA13156.09
Price (USD)/ Each
30.10
-
Chemical Identifiers
CAS693-02-7
IUPAC Namehex-1-yne
Molecular FormulaC6H10
InChI KeyCGHIBGNXEGJPQZ-UHFFFAOYSA-N
SMILESCCCCC#C
View more
Specifications Specification Sheet
Specification Sheet
Identification (FTIR)Conforms
Refractive Index1.3970-1.4010 @ 20?C
Appearance (Color)Clear colorless to pale yellow
Assay (GC)≥98.0%
FormLiquid
1-Hexyne is used in the preparation of tricyclic isoindolinone scaffold by undergoing hydrozirconation and ring-closing metathesis. It is used to prepare trans-1,2-bis(5-thianthreniumyl)alkene tetrafluoroborate by reacting with thianthrene cation radical tetrafluoroborate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Hexyne is used in the preparation of tricyclic isoindolinone scaffold by undergoing hydrozirconation and ring-closing metathesis. It is used to prepare trans-1,2-bis(5-thianthreniumyl)alkene tetrafluoroborate by reacting with thianthrene cation radical tetrafluoroborate.
Solubility
Miscible with organic solvents. Immiscible with water.
Notes
Incompatible with strong oxidizing agents, strong reducing agents, strong acids and strong bases.
1-Hexyne is used in the preparation of tricyclic isoindolinone scaffold by undergoing hydrozirconation and ring-closing metathesis. It is used to prepare trans-1,2-bis(5-thianthreniumyl)alkene tetrafluoroborate by reacting with thianthrene cation radical tetrafluoroborate.
Solubility
Miscible with organic solvents. Immiscible with water.
Notes
Incompatible with strong oxidizing agents, strong reducing agents, strong acids and strong bases.
RUO – Research Use Only
General References:
- The dilithiation of 1-alkynes with n-BuLi, followed by alkylation at the 3-position, is illustrated for 1-hexyne: Org. Synth. Coll., 6, 595 (1988). For [2 + 2] cycloaddition with dichloroketene to give a substituted cyclobutenone, see: Org. Synth. Coll., 8, 82 (1993).
- Van, D. D.; Hosokawa, T.; Saito, M.; Horiuchi, Y.; Matsuoka, M. A heterogeneous mesoporous silica-supported cyclopentadienyl ruthenium(II) complex catalyst for selective hydrosilylation of 1-hexyne at room temperature. Appl. Catal., A. 2015, 503, 203-208.
- Saito, M.; Watanabe, T.; Kamegawa, T.; Horiuchi, Y.; Matsuoka, M. Construction of an organoruthenium complex (-[biphRuCp]PF6-) within a biphenylene-bridged inorganic-organic hybrid mesoporous material, and its catalytic activity in the selective hydrosilylation of 1-hexyne. Res. Chem. Intermed. 2014, 40 (1), 105-113.