Search Thermo Fisher Scientific
Thermo Scientific Chemicals
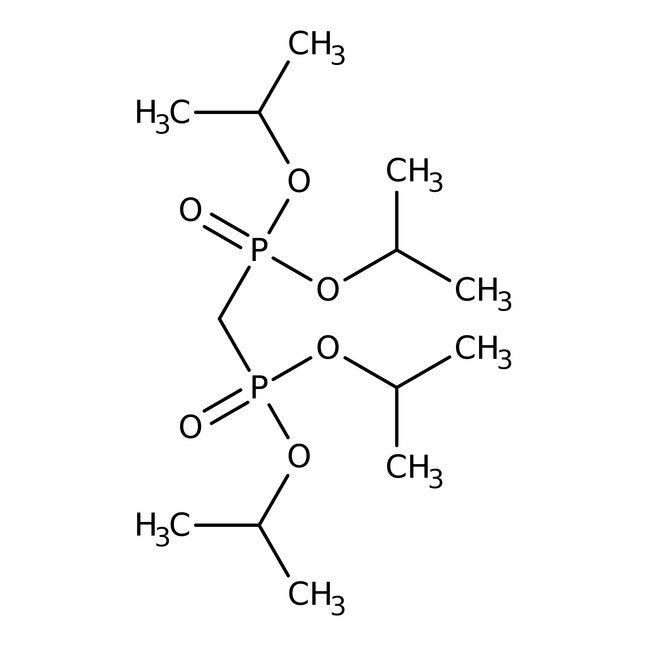
Tetraisopropyl methylenediphosphonate, 98%, Thermo Scientific Chemicals
Catalog number A13484.18
also known as A13484-18
Price (USD)/ Each
178.65
Online exclusive
198.00 Save 19.35 (10%)
-
Quantity:
50 g
Price (USD)/ Each
178.65
Online exclusive
198.00 Save 19.35 (10%)
Tetraisopropyl methylenediphosphonate, 98%, Thermo Scientific Chemicals
Catalog numberA13484.18
Price (USD)/ Each
178.65
Online exclusive
198.00 Save 19.35 (10%)
-
Chemical Identifiers
CAS1660-95-3
IUPAC Namebis(propan-2-yl) {[bis(propan-2-yloxy)phosphoryl]methyl}phosphonate
Molecular FormulaC13H30O6P2
InChI KeyODTQUKVFOLFLIQ-UHFFFAOYSA-N
SMILESCC(C)OP(=O)(CP(=O)(OC(C)C)OC(C)C)OC(C)C
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥97.5%
Refractive Index1.4320-1.4360 @ 20?C
Identification (FTIR)Conforms
FormLiquid
Appearance (Color)Clear colorless to pale yellow
Tetraisopropyl methylenediphosphonate is used in the preparation of AZT 5’-Triphosphate compounds which display inhibitory effects on HIV-1 reverse transcrpitase. It is also used in the preparation of E2-bisphosphonate conjugates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetraisopropyl methylenediphosphonate is used in the preparation of AZT 5’-Triphosphate compounds which display inhibitory effects on HIV-1 reverse transcrpitase. It is also used in the preparation of E2-bisphosphonate conjugates.
Solubility
Insoluble in water.
Notes
Store in cool, dry conditions in well sealed containers. Keep container tightly closed.
Tetraisopropyl methylenediphosphonate is used in the preparation of AZT 5’-Triphosphate compounds which display inhibitory effects on HIV-1 reverse transcrpitase. It is also used in the preparation of E2-bisphosphonate conjugates.
Solubility
Insoluble in water.
Notes
Store in cool, dry conditions in well sealed containers. Keep container tightly closed.
RUO – Research Use Only
General References:
- Richard D. Chambers.; John Hutchinson. Elemental fluorine. Part 91: Catalysis of the direct fluorination of 2-substituted carbonyl compounds2. Journal of Fluorine Chemistry. 1998, 92, (1), 45-52.
- Andrzej W. Trochimczuk. Novel ion-exchange/coordination resin with carboxyethyl phosphonate ligands. European Polymer Journal. 1998, 34, (7), 1047-1051.
- Horner-Wadsworth-Emmons olefination (see Appendix 1) with carbonyl compounds yields vinylphosphonates: Synth. Commun., 10, 299 (1980). A synthesis of allenes via a double HWE olefination has been described: Tetrahedron, 58, 83 (2002):
- gem-Bisphosphonates have application in bone tissue therapy: Synthesis, 661 (1991).