Search Thermo Fisher Scientific
Thermo Scientific Chemicals
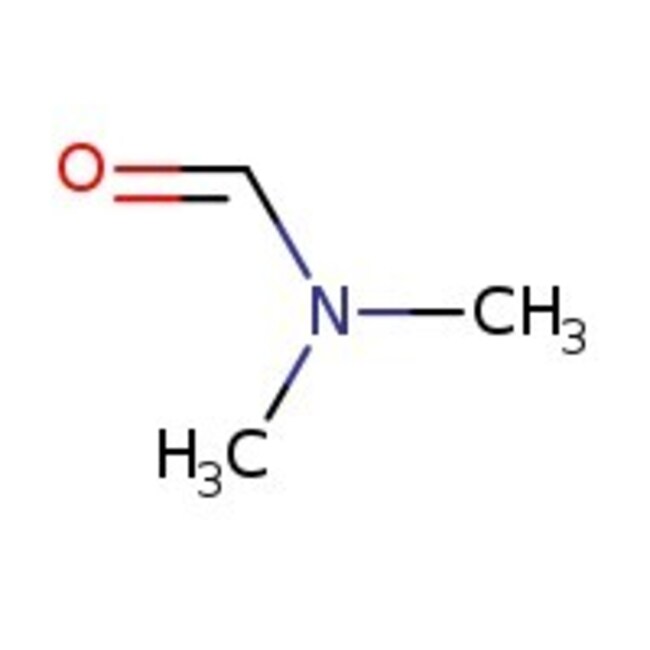
N,N-Dimethylformamide, 99%, Thermo Scientific Chemicals
Catalog number: A13547.0F
2500 mL, Each

Thermo Scientific Chemicals
N,N-Dimethylformamide, 99%, Thermo Scientific Chemicals
Catalog number: A13547.0F
2500 mL, Each
Quantity
Have Questions?
Chemical Identifiers
CAS
68-12-2
IUPAC Name
N,N-dimethylformamide
Molecular Formula
C3H7NO
InChI Key
ZMXDDKWLCZADIW-UHFFFAOYSA-N
SMILES
CN(C)C=O
Specifications
Refractive Index
1.4290-1.4320 @ 20°C or 1.4265-1.4295 @ 25°C
Form
Liquid
Identification (FTIR)
Conforms
Appearance (Color)
Clear colorless
Assay (GC)
≥98.5%
Description
N, N-Dimethylformamide is commonly used as a solvent. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N, N-Dimethylformamide is commonly used as a solvent. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
Solubility
Miscible with water and most organic solvents.
Notes
Reaction with sodium hydride gives exothermic decomposition at low temperatures. Keep container tightly closed in a dry and well-ventilated place.
N, N-Dimethylformamide is commonly used as a solvent. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
Solubility
Miscible with water and most organic solvents.
Notes
Reaction with sodium hydride gives exothermic decomposition at low temperatures. Keep container tightly closed in a dry and well-ventilated place.
WARNING: Cancer – www.P65Warnings.ca.gov
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text