Search Thermo Fisher Scientific
Thermo Scientific Chemicals
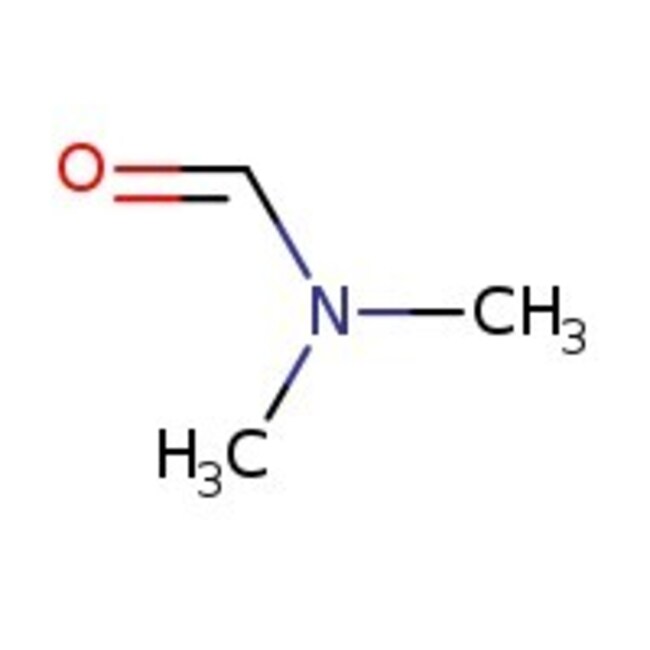
N,N-Dimethylformamide, 99%, Thermo Scientific Chemicals
Catalog number A13547.AK
Price (USD)/ Each
25.65
Special Offer
Online exclusive
Ends: 31-Dec-2024
30.00 Save 4.35 (15%)
-
Quantity:
250 mL
Price (USD)/ Each
25.65
Special Offer
Online exclusive
Ends: 31-Dec-2024
30.00 Save 4.35 (15%)
N,N-Dimethylformamide, 99%, Thermo Scientific Chemicals
Catalog numberA13547.AK
Price (USD)/ Each
25.65
Special Offer
Online exclusive
Ends: 31-Dec-2024
30.00 Save 4.35 (15%)
-
Chemical Identifiers
CAS68-12-2
IUPAC NameN,N-dimethylformamide
Molecular FormulaC3H7NO
InChI KeyZMXDDKWLCZADIW-UHFFFAOYSA-N
SMILESCN(C)C=O
View more
Specifications Specification Sheet
Specification Sheet
Refractive Index1.4290-1.4320 @ 20°C or 1.4265-1.4295 @ 25°C
FormLiquid
Identification (FTIR)Conforms
Appearance (Color)Clear colorless
Assay (GC)≥98.5%
View more
N, N-Dimethylformamide is commonly used as a solvent. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N, N-Dimethylformamide is commonly used as a solvent. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
Solubility
Miscible with water and most organic solvents.
Notes
Reaction with sodium hydride gives exothermic decomposition at low temperatures. Keep container tightly closed in a dry and well-ventilated place.
N, N-Dimethylformamide is commonly used as a solvent. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
Solubility
Miscible with water and most organic solvents.
Notes
Reaction with sodium hydride gives exothermic decomposition at low temperatures. Keep container tightly closed in a dry and well-ventilated place.
WARNING: Cancer – www.P65Warnings.ca.gov
RUO – Research Use Only
General References:
- Dipolar aprotic solvent, in which anions have enhanced nucleophilicity, compare Dimethyl sulfoxide, A13280. Effective solvent for a vast range of reactions.
- CAUTION! Thermal runaway reactions can occur with NaH; see Sodium hydride, 13431.
- In combination with POCl3, (COCl)2, etc., generates an iminium chloride intermediate for the formylation of reactive aromatic nuclei by the Vilsmeier reaction:
- Compare also N-Methyl formanilide, A11829, and preformed Vilsmeier reagent (Chloromethyl ene) dimethyl ammonium chloride, B24172. Monograph: Synthesis Using Vilsmeier Reagents, C. M. Marson, P. R. Giles, CRC Press, Boca Raton, FL (1994). Formylation can be extended to less active substrates, e.g. acenaphthene or mesitylene, with the DMF-triflic anhydride reagent: J. Chem. Soc., Chem. Commun., 1571 (1990). Under Vilsmeier conditions, electron-rich alkenes can be converted to ɑß-unsaturated aldehydes; see, e.g.: Synthesis, 752 (1976); and ketones to ß-chloroenals: Synthesis, 496 (1985); Org. Synth. Coll., 5, 215 (1973).
- Vilsmeier conditions also promote dehydrations, e.g. in cyclodehydration of hydroxyphenols to give cyclic ethers, as an efficient alternative to the Mitsunobu reaction: J. Chem. Soc., Perkin 1, 2249 (1996); J. Org. Chem., 59, 4346 (1994):
- DMF can also be used for formylation with alkyllithium or Grignard reagents: Synthesis, 228 (1984).
- In aqueous DMF, tosylate esters undergo formolysis to formate esters: Synth. Commun., 26, 1031 (1996).
- For the free-radical formylation of perfluoroalkyl iodides, see Zinc-copper couple, L09811.