Search Thermo Fisher Scientific
Thermo Scientific Chemicals
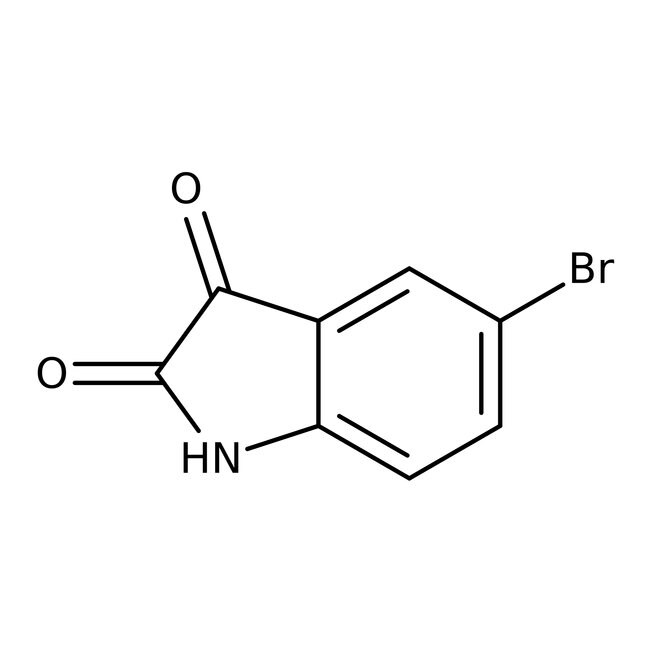
5-Bromoisatin, 90+%, Thermo Scientific Chemicals
Catalog number A13641.18
also known as A13641-18
Price (USD)/ Each
79.00
-
Quantity:
50 g
Price (USD)/ Each
79.00
5-Bromoisatin, 90+%, Thermo Scientific Chemicals
Catalog numberA13641.18
Price (USD)/ Each
79.00
-
Chemical Identifiers
CAS87-48-9
IUPAC Name5-bromo-2,3-dihydro-1H-indole-2,3-dione
Molecular FormulaC8H4BrNO2
InChI KeyMBVCESWADCIXJN-UHFFFAOYSA-N
SMILESBrC1=CC=C2NC(=O)C(=O)C2=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Orange
Assay (HPLC)≥90.0%
Water Content (Karl Fischer Titration)≤5%
FormPowder
Identification (FTIR)Conforms
5-Bromoisatin is used in the synthesis of N-derivatives of 5-bromoisatin, N-substituted pyrroles, linear polyaryleneoxindoles, 5-bromodioxindole, cinchoninic acid derivatives, 3-hydroxyoxindole, S-benzyldithiocarbazate Schiff Bases, 5-bromooxindole and Morita-Baylis-Hillman adducts of isatin derivatives. It is an indole derivative.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
5-Bromoisatin is used in the synthesis of N-derivatives of 5-bromoisatin, N-substituted pyrroles, linear polyaryleneoxindoles, 5-bromodioxindole, cinchoninic acid derivatives, 3-hydroxyoxindole, S-benzyldithiocarbazate Schiff Bases, 5-bromooxindole and Morita-Baylis-Hillman adducts of isatin derivatives. It is an indole derivative.
Solubility
Soluble in N,N dimethylformamide.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
5-Bromoisatin is used in the synthesis of N-derivatives of 5-bromoisatin, N-substituted pyrroles, linear polyaryleneoxindoles, 5-bromodioxindole, cinchoninic acid derivatives, 3-hydroxyoxindole, S-benzyldithiocarbazate Schiff Bases, 5-bromooxindole and Morita-Baylis-Hillman adducts of isatin derivatives. It is an indole derivative.
Solubility
Soluble in N,N dimethylformamide.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Sumpter WC. The Structures of the Bromodioxindoles of Baeyer and Knop. J. Am. Chem. Soc. 1945, 67 (7), 1140-1141.
- Sarkis GY. Synthesis and spectral data for cinchoninic acids. J. Chem. Eng. Data. 1972, 17 (3), 388-391.