Search Thermo Fisher Scientific
Thermo Scientific Chemicals
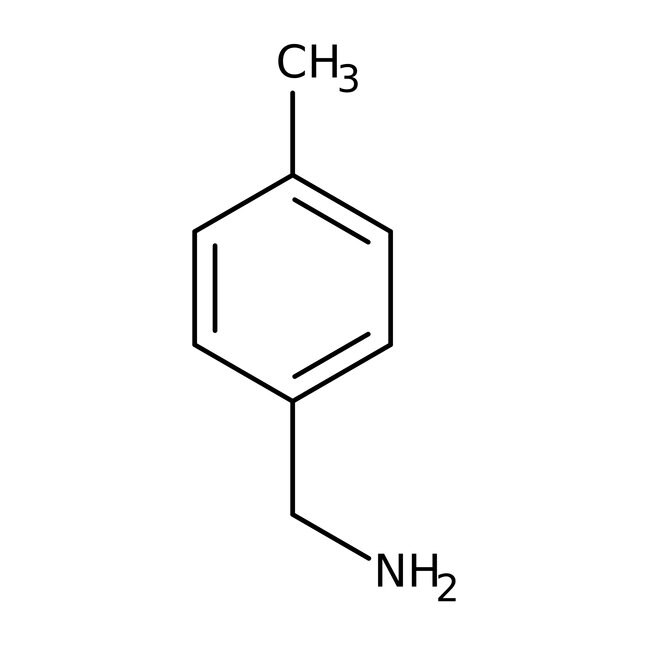
4-Methylbenzylamine, 98%, Thermo Scientific Chemicals
Catalog number A13768.22
also known as A13768-22
Price (USD)/ Each
164.00
-
Quantity:
100 g
Price (USD)/ Each
164.00
4-Methylbenzylamine, 98%, Thermo Scientific Chemicals
Catalog numberA13768.22
Price (USD)/ Each
164.00
-
Chemical Identifiers
CAS104-84-7
IUPAC Name1-(4-methylphenyl)methanamine
Molecular FormulaC8H11N
InChI KeyHMTSWYPNXFHGEP-UHFFFAOYSA-N
SMILESCC1=CC=C(CN)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
Refractive Index1.5330-1.5370 @ 20?C
Assay (GC)≥97.5%
FormLiquid
Identification (FTIR)Conforms
The reactions of 1-hexanol with benzylamine and with 4-methylbenzylamine led to the corresponding imines in moderate yields. 4-methylbenzylamine in ethanol/water solution (1:4) as simultaneously absorbing and buffering background electrolyte with detection at 210 nm was found suitable for determination of the individual compounds in determination by capillary zone electrophoresis with indirect detection. Synthesis of benzimidazoles uses 4-methylbenzylamine (R 1 =4-MeBn) and 4-chlorophenyl isothiocyanate (R 2 =4-ClPh) as building blocks.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
The reactions of 1-hexanol with benzylamine and with 4-methylbenzylamine led to the corresponding imines in moderate yields. 4-methylbenzylamine in ethanol/water solution (1:4) as simultaneously absorbing and buffering background electrolyte with detection at 210 nm was found suitable for determination of the individual compounds in determination by capillary zone electrophoresis with indirect detection. Synthesis of benzimidazoles uses 4-methylbenzylamine (R 1 =4-MeBn) and 4-chlorophenyl isothiocyanate (R 2 =4-ClPh) as building blocks.
Solubility
Slightly soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
The reactions of 1-hexanol with benzylamine and with 4-methylbenzylamine led to the corresponding imines in moderate yields. 4-methylbenzylamine in ethanol/water solution (1:4) as simultaneously absorbing and buffering background electrolyte with detection at 210 nm was found suitable for determination of the individual compounds in determination by capillary zone electrophoresis with indirect detection. Synthesis of benzimidazoles uses 4-methylbenzylamine (R 1 =4-MeBn) and 4-chlorophenyl isothiocyanate (R 2 =4-ClPh) as building blocks.
Solubility
Slightly soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
General References:
- Viktor Krchňák,; Jennifer Smith,; Josef Vágner. A solid phase traceless synthesis of 2-arylaminobenzimidazoles. Tetrahedron Letters. 2001, 42 (9), 1627-1630.
- Nad’a Reichová,; Jiří Pazourek,; Pavla Polášková andJosef Havel. Electrophoretic behavior of adamantane derivatives possessing antiviral activity and their determination by capillary zone electrophoresis with indirect detection. Electrophoresis. 2002, 23 (2), 259-262.