Search Thermo Fisher Scientific
Thermo Scientific Chemicals
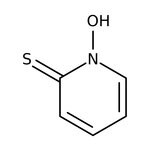
2-Mercaptopyridine N-oxide, 99%, Thermo Scientific Chemicals
Catalog number: A14152.06
5 g, Each


Thermo Scientific Chemicals
2-Mercaptopyridine N-oxide, 99%, Thermo Scientific Chemicals
Catalog number: A14152.06
5 g, Each
Quantity
Catalog number: A14152.06
also known as A14152-06
Price (USD)
80.00
Each
Quantity
-
Have Questions?
Chemical Identifiers
CAS
1121-31-9
IUPAC Name
1-hydroxy-1,2-dihydropyridine-2-thione
Molecular Formula
C5H5NOS
InChI Key
YBBJKCMMCRQZMA-UHFFFAOYSA-N
SMILES
ON1C=CC=CC1=S
Specifications
Form
Crystals or powder or crystalline powder
Appearance (Color)
White to cream to yellow to green to grey
Assay (Aqueous acid-base Titration)
≥98.5 to ≤101.5%
Assay (GC)
≥98.5%
Melting Point (clear melt)
67.0-74.0?C
Description
2-Mercaptopyridine N-oxide is used in ratiometric fluorescence imaging of intracellular zinc ions in mammalian cultures. It is used in the radical coupling of benzoyl derivatives of 2-deoxy-D-ribose, with heterocyclic bases to give C-nucleosides. It acts as a ligand and form coordination complexes with palladium chloride. It is a precursor to O-acyl thiohydroxamates. Further, it serves as an antifungal agent. In addition to this, it is used in the preparation of 1-oxa-2-oxo-3-thia-indolizinium chloride by reaction with carbonyl dichloride.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Mercaptopyridine N-oxide is used in ratiometric fluorescence imaging of intracellular zinc ions in mammalian cultures. It is used in the radical coupling of benzoyl derivatives of 2-deoxy-D-ribose, with heterocyclic bases to give C-nucleosides. It acts as a ligand and form coordination complexes with palladium chloride. It is a precursor to O-acyl thiohydroxamates. Further, it serves as an antifungal agent. In addition to this, it is used in the preparation of 1-oxa-2-oxo-3-thia-indolizinium chloride by reaction with carbonyl dichloride.
Solubility
Soluble in tris-hydrochloric acid.
Notes
Incompatible with strong oxidizing agents and strong bases.
2-Mercaptopyridine N-oxide is used in ratiometric fluorescence imaging of intracellular zinc ions in mammalian cultures. It is used in the radical coupling of benzoyl derivatives of 2-deoxy-D-ribose, with heterocyclic bases to give C-nucleosides. It acts as a ligand and form coordination complexes with palladium chloride. It is a precursor to O-acyl thiohydroxamates. Further, it serves as an antifungal agent. In addition to this, it is used in the preparation of 1-oxa-2-oxo-3-thia-indolizinium chloride by reaction with carbonyl dichloride.
Solubility
Soluble in tris-hydrochloric acid.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
Figures
Documents & Downloads
Certificates
Search by lot number or partial lot number
Frequently asked questions (FAQs)
Citations & References
Search citations by name, author, journal title or abstract text