Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Benzoic anhydride, 98%, Thermo Scientific Chemicals
Catalog number A14269.0B
also known as A14269-0B
Price (USD)/ Each
255.65
Online exclusive
284.00 Save 28.35 (10%)
-
Quantity:
1000 g
Price (USD)/ Each
255.65
Online exclusive
284.00 Save 28.35 (10%)
Benzoic anhydride, 98%, Thermo Scientific Chemicals
Catalog numberA14269.0B
Price (USD)/ Each
255.65
Online exclusive
284.00 Save 28.35 (10%)
-
Chemical Identifiers
CAS93-97-0
IUPAC Namebenzoyl benzoate
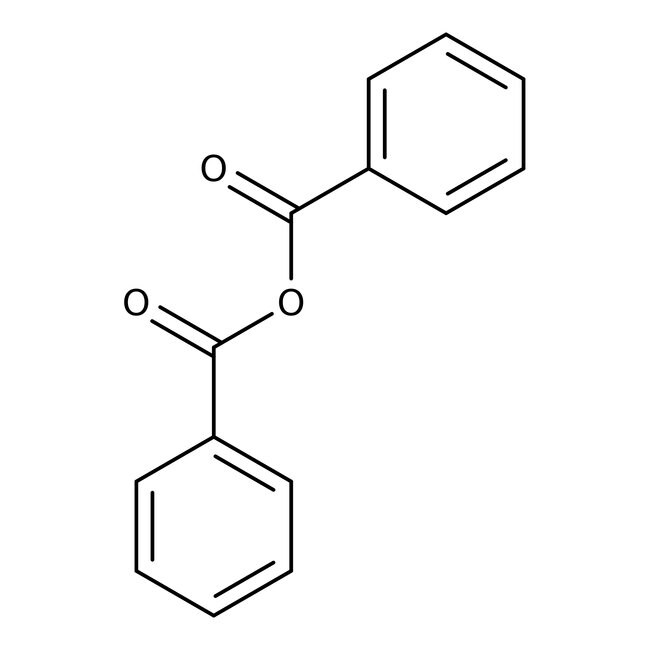
Molecular FormulaC14H10O3
InChI KeyCHIHQLCVLOXUJW-UHFFFAOYSA-N
SMILESO=C(OC(=O)C1=CC=CC=C1)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream
FormPowder or crystalline powder or crystals
Assay (chromatographic, GC or HPLC)≥97.5%
Identification (FTIR)Conforms
Melting Point (clear melt)38-45°C
Benzoic anhydride is used as a benzoylating agent in the manufacture of pharmaceuticals, dyes and intermediates. It serves as an arylation agent in the Heck reaction. It is also used to get benzoic acid through dehydration. Further, it is used to prepare furan-2-yl-phenyl-methanone, which is similar to Friedel-Crafts acylation reaction. In addition to this, it is used for the synthesis of carboxylic esters and lactones.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Benzoic anhydride is used as a benzoylating agent in the manufacture of pharmaceuticals, dyes and intermediates. It serves as an arylation agent in the Heck reaction. It is also used to get benzoic acid through dehydration. Further, it is used to prepare furan-2-yl-phenyl-methanone, which is similar to Friedel-Crafts acylation reaction. In addition to this, it is used for the synthesis of carboxylic esters and lactones.
Solubility
Soluble in alcohol, ether, chloroform, acetone, ethyl acetate, benzene, toluene, glacial acetic acid, acetic anhydride and ethanol. Slightly soluble in benzene and acetone. Insoluble in water.
Notes
Moisture sensitive. Incompatible with strong acids, strong oxidizing agents and strong bases.
Benzoic anhydride is used as a benzoylating agent in the manufacture of pharmaceuticals, dyes and intermediates. It serves as an arylation agent in the Heck reaction. It is also used to get benzoic acid through dehydration. Further, it is used to prepare furan-2-yl-phenyl-methanone, which is similar to Friedel-Crafts acylation reaction. In addition to this, it is used for the synthesis of carboxylic esters and lactones.
Solubility
Soluble in alcohol, ether, chloroform, acetone, ethyl acetate, benzene, toluene, glacial acetic acid, acetic anhydride and ethanol. Slightly soluble in benzene and acetone. Insoluble in water.
Notes
Moisture sensitive. Incompatible with strong acids, strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Reagent, in the presence of excess pyridine, for decarboxylation of ɑ-keto acids, giving good yields of aldehydes without Perkin condensation: J. Am. Chem. Soc., 87, 3780 (1965).
- In the presence of RuCl3, tertiary amines are dealkylated and benzoylated to give N,N-disubstituted benzamides: J. Organomet. Chem., 252, C9 (1983).
- Hou, J.; Xie, J. H.; Zhou, Q. L. Palladium-Catalyzed Hydrocarboxylation of Alkynes with Formic Acid. Angew. Chem. Int. Ed. 2015, 127 (21), 6400-6403.
- Sun, L.; Fan, Z.; Wang, Y.; Huang, Y.; Schmidt, M.; Zhang, M. Tunable synthesis of self-assembled cyclic peptide nanotubes and nanoparticles. Soft Matter 2015, 11 (19), 3822-3832.