Search Thermo Fisher Scientific
Thermo Scientific Chemicals
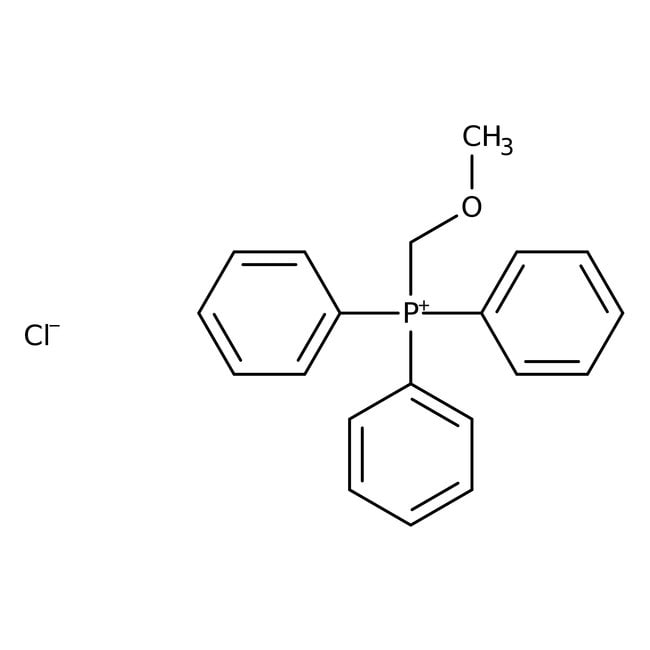
(Methoxymethyl)triphenylphosphonium chloride, 98+%, Thermo Scientific Chemicals
Catalog number A14380.22
also known as A14380-22
Price (USD)/ Each
128.65
Online exclusive
143.00 Save 14.35 (10%)
-
Quantity:
100 g
Price (USD)/ Each
128.65
Online exclusive
143.00 Save 14.35 (10%)
(Methoxymethyl)triphenylphosphonium chloride, 98+%, Thermo Scientific Chemicals
Catalog numberA14380.22
Price (USD)/ Each
128.65
Online exclusive
143.00 Save 14.35 (10%)
-
Chemical Identifiers
CAS4009-98-7
IUPAC Name(methoxymethyl)triphenylphosphanium chloride
Molecular FormulaC20H20ClOP
InChI KeySJFNDMHZXCUXSA-UHFFFAOYSA-M
SMILES[Cl-].COC[P+](C1=CC=CC=C1)(C1=CC=CC=C1)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Water Content (Karl Fischer Titration)<2.0%
Appearance (Color)White to very pale cream
Assay (Titration ex Chloride)≥98.0 to ≤102.0% (dry wt basis)
FormPowder or crystals or crystalline powder
Identification (FTIR)Conforms
(Methoxymethyl)triphenylphosphonium chloride is used as a phase transfer catalyst and in the synthesis of taxol-A fragment. It is widely used in the synthesis of pharmaceutical product cephalotaxine, which is used as an antiviral and antitumor agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(Methoxymethyl)triphenylphosphonium chloride is used as a phase transfer catalyst and in the synthesis of taxol-A fragment. It is widely used in the synthesis of pharmaceutical product cephalotaxine, which is used as an antiviral and antitumor agent.
Solubility
Soluble in methanol and chloroform. It decomposes in water.
Notes
Hygroscopic . Incompatible with strong oxidizing agents.
(Methoxymethyl)triphenylphosphonium chloride is used as a phase transfer catalyst and in the synthesis of taxol-A fragment. It is widely used in the synthesis of pharmaceutical product cephalotaxine, which is used as an antiviral and antitumor agent.
Solubility
Soluble in methanol and chloroform. It decomposes in water.
Notes
Hygroscopic . Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Wittig reaction (see Appendix 1) of the derived phosphorane with aldehydes and ketones gives enol ethers, readily hydrolyzed to homologous aldehydes. For an example (adamantanone to adamantanecarboxaldehyde), see: Chem. Ber., 95, 2514 (1962). For use of the reaction in the synthesis of hydroisoquinoline AMPA antagonists, see: J. Med. Chem., 39, 2219 (1996). Reaction with chiral 2,3-epoxyaldehydes provides an enantioselective synthesis of (E)-4-hydroxyalkenes: J. Chem. Soc., Chem. Commun., 232 (1993).
- Lee, K.; Poudel, Y. B.; Glinkerman, C. M.; Boger, D. L. Total synthesis of dihydrolysergic acid and dihydrolysergol: development of a divergent synthetic strategy applicable to rapid assembly of D-ring analogs. Tetrahedron 2015, 71 (35), 5897-5905.
- Yu, Q.; Ma, S. An Enantioselective Synthesis of (R)-5,6-Octadecadienoic Acid. Eur. J. Org. Chem. 2015, 2015 (7), 1596-1601.