Search Thermo Fisher Scientific
Thermo Scientific Chemicals
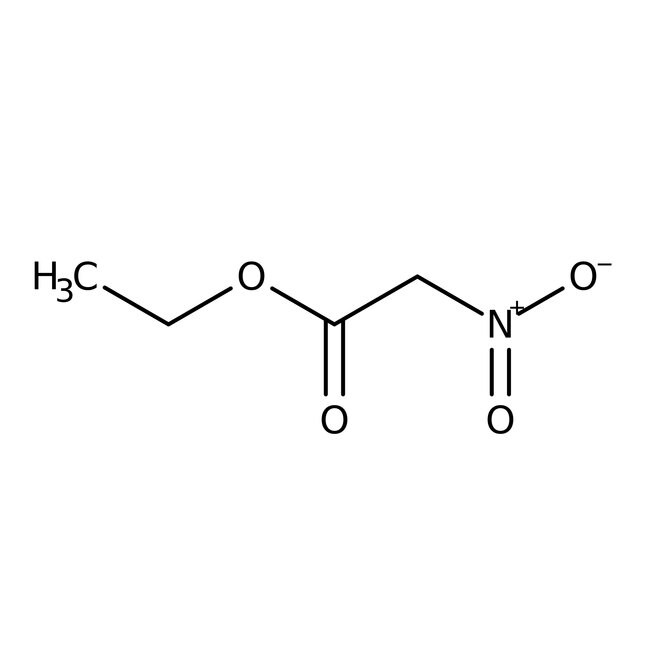
Ethyl nitroacetate, 97%, Thermo Scientific Chemicals
Catalog number A14433.22
also known as A14433-22
Price (USD)/ Each
785.00
-
Quantity:
100 g
Price (USD)/ Each
785.00
Ethyl nitroacetate, 97%, Thermo Scientific Chemicals
Catalog numberA14433.22
Price (USD)/ Each
785.00
-
Chemical Identifiers
CAS626-35-7
IUPAC Nameethyl 2-nitroacetate
Molecular FormulaC4H7NO4
InChI KeyFTKASJMIPSSXBP-UHFFFAOYSA-N
SMILESCCOC(=O)C[N+]([O-])=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
Identification (FTIR)Conforms
Assay (GC)≥96.0%
Refractive Index1.4220-1.4270 @ 20?C
FormLiquid
Ethyl nitroacetate has been used in the synthesis of γ-oxoacids via Michael addition reaction with α,β-unsaturated ketones, fuctionalization of C4-position on pyrimidine and C6-position on 2-deoxyguanosine to produce novel nucleosides, facile synthesis of α,α-diisobutylglycine, synthesis of DL-4,4-difluoroglutamic acid. It is also used as an intermediate in the preparation of unsubstituted amino acids.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl nitroacetate has been used in the synthesis of γ-oxoacids via Michael addition reaction with α,β-unsaturated ketones, fuctionalization of C4-position on pyrimidine and C6-position on 2′-deoxyguanosine to produce novel nucleosides, facile synthesis of α,α-diisobutylglycine, synthesis of DL-4,4-difluoroglutamic acid. It is also used as an intermediate in the preparation of unsubstituted amino acids.
Solubility
Slightly soluble in water. Soluble in chloroform and ethyl acetate.
Notes
Store away from bases and oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
Ethyl nitroacetate has been used in the synthesis of γ-oxoacids via Michael addition reaction with α,β-unsaturated ketones, fuctionalization of C4-position on pyrimidine and C6-position on 2′-deoxyguanosine to produce novel nucleosides, facile synthesis of α,α-diisobutylglycine, synthesis of DL-4,4-difluoroglutamic acid. It is also used as an intermediate in the preparation of unsubstituted amino acids.
Solubility
Slightly soluble in water. Soluble in chloroform and ethyl acetate.
Notes
Store away from bases and oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Maialen Aginagalde, et al. Formation of γ-oxoacids and 1H-pyrrol-2(5H)-ones from α,β-unsaturated ketones and ethyl nitroacetate.J. Org. Chem.,2010,75(21), 7435-8.
- Victor Timoshchuk. Functionalization of pyrimidine and purine nucleosides at C4 and C6: C-nucleophilic substitution of their C4- and C6-(1,2,4-triazol-1-yl) derivatives.Nucleosides Nucleotides Nucleic Acids.,2005,24(5-7), 1043-1046.
- In the presence of the Mitsunobu reagent (PPh3/DEAD) oxidizes alcohols to carbonyl compounds: Tetrahedron Lett., 22, 2295 (1981).
- Reacts with 2,3-epoxyaldehydes to give 3-ethoxycarbonyl-4-hydroxy-5-(1-hydroxyalkyl)-2-isoxazoline-2-oxides: J. Org. Chem., 55, 781 (1990):
- Similarly, reaction with ɑ-bromo enones gives 5-acyl-3-(ethoxycarbonyl)-2-isoxazoline-2-oxides by a tandem conjugate addition - ring closure: J. Org. Chem., 60, 6624 (1995).
- Conjugate addition to various enones, followed by reductive cyclization with Formamidinesulfinic acid, A11885, provides a convenient new route to pyrroles: Tetrahedron Lett., 36, 9469 (1995). For examples with reaction schemes, see 2-Benzyl idenecyclohexanone, L13434 and 3-Penten-2-one, L13031.
- For a review of the use of nitroacetic acid and its esters in organic synthesis, see: Synthesis, 666 (1979).