Search Thermo Fisher Scientific
Thermo Scientific Chemicals
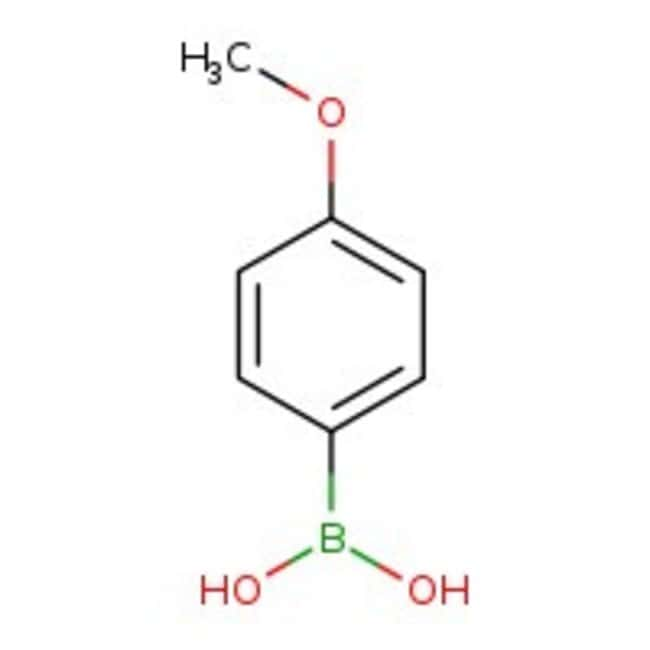
4-Methoxybenzeneboronic acid, 97+%, Thermo Scientific Chemicals
Catalog number A14462.06
also known as A14462-06
Price (USD)/ Each
96.50
-
Quantity:
5 g
Price (USD)/ Each
96.50
4-Methoxybenzeneboronic acid, 97+%, Thermo Scientific Chemicals
Catalog numberA14462.06
Price (USD)/ Each
96.50
-
Chemical Identifiers
CAS5720-07-0
IUPAC Name(4-methoxyphenyl)boronic acid
Molecular FormulaC7H9BO3
InChI KeyVOAAEKKFGLPLLU-UHFFFAOYSA-N
SMILESCOC1=CC=C(C=C1)B(O)O
View more
Specifications Specification Sheet
Specification Sheet
Assay (Aqueous acid-base Titration)≥97.0%
Assay (HPLC)≥97.0%
Identification (FTIR)Conforms
Proton NMRConforms to structure
Appearance (Color)White to pale cream to pale brown
View more
4-Methoxybenzeneboronic acid is used for Suzuki-Miyaura cross-coupling reactions, Pd-catalyzed direct arylation, Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, Palladium-catalyzed stereoselective Heck-type reaction, Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, Ruthenium catalyzed direct arylation, Rh-catalyzed asymmetric conjugate addition, Ligand-free copper-catalyzed coupling.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Methoxybenzeneboronic acid is used for Suzuki-Miyaura cross-coupling reactions, Pd-catalyzed direct arylation, Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, Palladium-catalyzed stereoselective Heck-type reaction, Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, Ruthenium catalyzed direct arylation, Rh-catalyzed asymmetric conjugate addition, Ligand-free copper-catalyzed coupling.
Solubility
Soluble in dimethyl sulfoxide and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
4-Methoxybenzeneboronic acid is used for Suzuki-Miyaura cross-coupling reactions, Pd-catalyzed direct arylation, Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, Palladium-catalyzed stereoselective Heck-type reaction, Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, Ruthenium catalyzed direct arylation, Rh-catalyzed asymmetric conjugate addition, Ligand-free copper-catalyzed coupling.
Solubility
Soluble in dimethyl sulfoxide and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
RUO – Research Use Only
General References:
- S.A. Barker.; A.K. Chopra.; B.W. Hatt.; P.J. Somers. The interaction of areneboronic acids with monosaccharides . Carbohydrate Research. 1973, 26 (1), 33-40.
- S.A. Barker, B.W. Hatt, P.J. Somers. The effect of areneboronic acids on the alkaline conversion of d-glucose into d-fructose. Carbohydrate Research . 1973, 26 (1), 41-53.
- GC reagent for diols: J. Chromat., 158, 33 (1978), 186, 307 (1979).
- For an illustrative example of the silver oxide promoted Suzuki coupling with sensitive ɑ-halo enones, under extremely mild conditions, see: Org. Synth., 75, 69 (1997):