Search Thermo Fisher Scientific
Thermo Scientific Chemicals
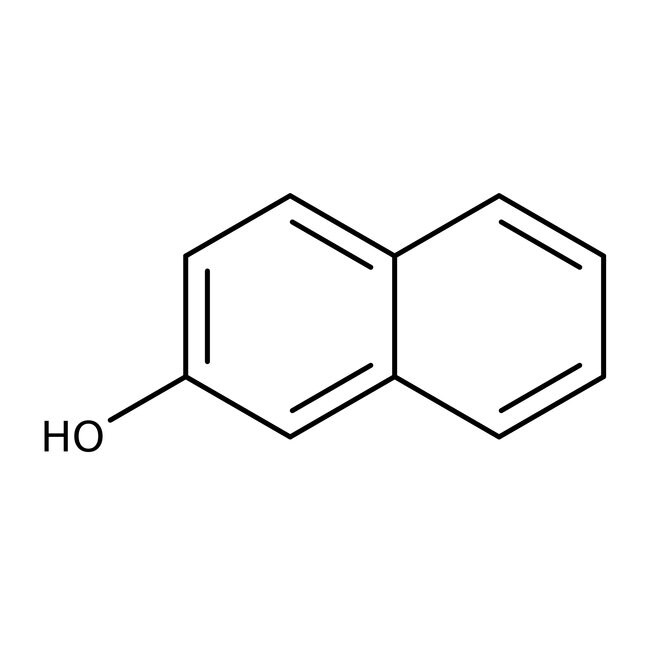
2-Naphthol, 98+%, Thermo Scientific Chemicals
Catalog number A14564.30
also known as A14564-30
Price (USD)/ Each
30.65
Online exclusive
33.80 Save 3.15 (9%)
-
Quantity:
250 g
Price (USD)/ Each
30.65
Online exclusive
33.80 Save 3.15 (9%)
2-Naphthol, 98+%, Thermo Scientific Chemicals
Catalog numberA14564.30
Price (USD)/ Each
30.65
Online exclusive
33.80 Save 3.15 (9%)
-
Chemical Identifiers
CAS135-19-3
IUPAC Namenaphthalen-2-ol
Molecular FormulaC10H8O
InChI KeyJWAZRIHNYRIHIV-UHFFFAOYSA-N
SMILESOC1=CC=C2C=CC=CC2=C1
View more
Specifications Specification Sheet
Specification Sheet
FormFlakes
Assay (GC)≥98.0%
Melting Point (clear melt)118.0-125.0?C
Appearance (Color)Cream to pale brown or pale pink
Identification (FTIR)Conforms
2-Naphthol can be used as a fluorescent indicator. It can be also used in the production of dyes and in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Naphthol can be used as a fluorescent indicator. It can be also used in the production of dyes and in organic synthesis.
Solubility
Partly miscible in water (0.76 g/L).
Notes
It is sensitive to light. Incompatible with oxidizing agents.
2-Naphthol can be used as a fluorescent indicator. It can be also used in the production of dyes and in organic synthesis.
Solubility
Partly miscible in water (0.76 g/L).
Notes
It is sensitive to light. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- M. Panizza; P.A.Michaud, G.Cerisola; Ch. Comninellis. Anodic oxidation of 2-naphthol at boron-doped diamond electrodes. Journal of Electroanalytical Chemistry. 2001, 507, (1-2),206-214
- William R.Laws; Ludwig. Brand. Analysis of two-state excited-state reactions. The fluorescence decay of 2-naphthol. J. Phys. Chem. 1979, 83, (7),795-802