Search Thermo Fisher Scientific
Thermo Scientific Chemicals
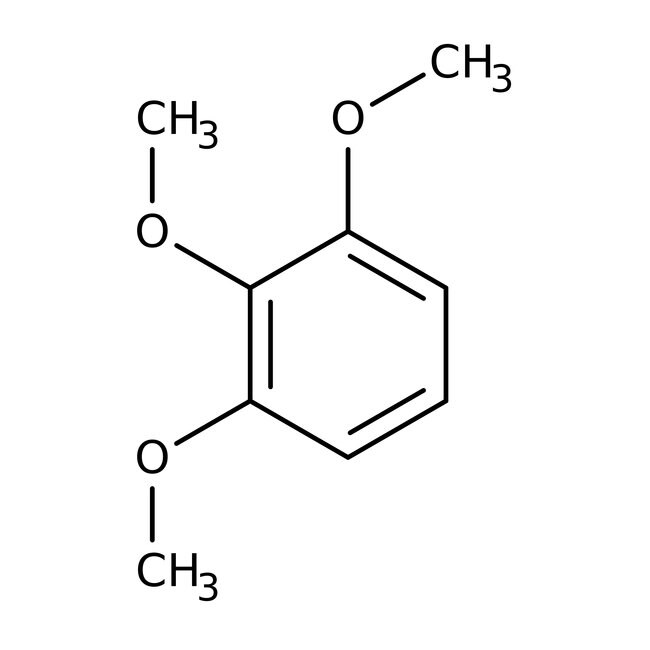
1,2,3-Trimethoxybenzene, 98+%, Thermo Scientific Chemicals
Catalog number A14572.14
also known as A14572-14
Price (USD)/ Each
34.40
-
Quantity:
25 g
Price (USD)/ Each
34.40
1,2,3-Trimethoxybenzene, 98+%, Thermo Scientific Chemicals
Catalog numberA14572.14
Price (USD)/ Each
34.40
-
Chemical Identifiers
CAS634-36-6
IUPAC Name1,2,3-trimethoxybenzene
Molecular FormulaC9H12O3
InChI KeyCRUILBNAQILVHZ-UHFFFAOYSA-N
SMILESCOC1=CC=CC(OC)=C1OC
View more
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder or fused/lumpy solid
Melting Point (clear melt)42.0-48.0?C
Appearance (Color)White to cream or pale brown
Assay (GC)≥98.0%
1,2,3-Trimethoxybenzene was used to study the effect of solvent on photo induced electron-transfer reactions, on condensation with 2,4-diamino-5-(hydroxymethyl)pyrimidine yields 2,4-diamino-5-(2,3,4-trimethoxybenzyl)pyrimidine.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,2,3-Trimethoxybenzene was used to study the effect of solvent on photo induced electron-transfer reactions, on condensation with 2,4-diamino-5-(hydroxymethyl)pyrimidine yields 2,4-diamino-5-(2,3,4-trimethoxybenzyl)pyrimidine.
Solubility
Insoluble in water.
Notes
Store in cool, dry conditions in a well sealed container. Incompatible with oxidizing agents.
1,2,3-Trimethoxybenzene was used to study the effect of solvent on photo induced electron-transfer reactions, on condensation with 2,4-diamino-5-(hydroxymethyl)pyrimidine yields 2,4-diamino-5-(2,3,4-trimethoxybenzyl)pyrimidine.
Solubility
Insoluble in water.
Notes
Store in cool, dry conditions in a well sealed container. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- A Stuart; T Paterson; B Roth; E Aig. 2,4-diamino-5-benzylpyrimidines and analogues as antibacterial agents. 6. A one-step synthesis of new trimethoprim derivatives and activity analysis by molecular modeling. Journal of Medicinal Chemistry. 1983, 26 (5), 667-673.
- Liming Zhang; Göran Gellerstedt. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. JMagnetic Resonance in Chemistry. 2007, 45 (1), 37-45.
- Direct dilithiation with n-BuLi/TMEDA gave poor results, but a sequence involving monolithiation, silylation and further lithiation at the 6-position gave the 4,6-disilyl derivative in 62% yield: J. Org. Chem., 49, 4657 (1984).