Search Thermo Fisher Scientific
Thermo Scientific Chemicals
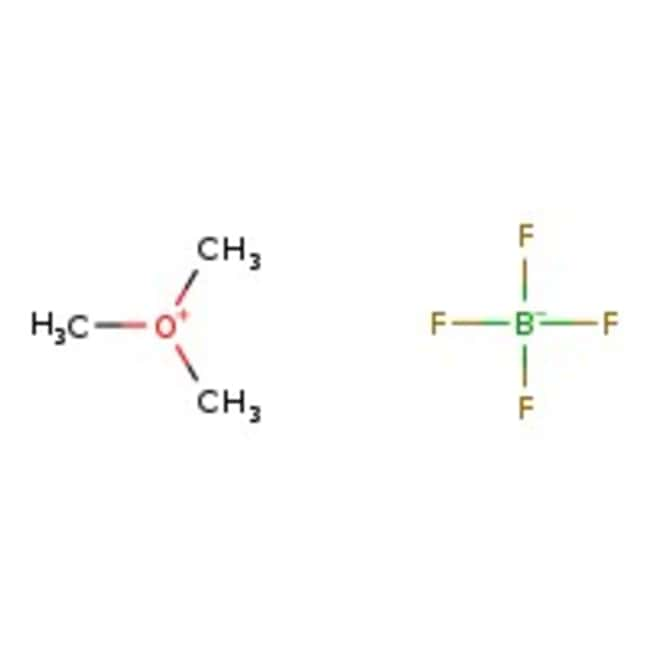
Trimethyloxonium tetrafluoroborate, 96%, Thermo Scientific Chemicals
Catalog number A15175.09
also known as A15175-09
Price (USD)/ Each
67.65
Online exclusive
75.00 Save 7.35 (10%)
-
Quantity:
10 g
Price (USD)/ Each
67.65
Online exclusive
75.00 Save 7.35 (10%)
Trimethyloxonium tetrafluoroborate, 96%, Thermo Scientific Chemicals
Catalog numberA15175.09
Price (USD)/ Each
67.65
Online exclusive
75.00 Save 7.35 (10%)
-
Chemical Identifiers
CAS420-37-1
IUPAC Nametetrafluoroboranuide; trimethyloxidanium
Molecular FormulaC3H9BF4O
InChI KeyCZVZBKHWOFJNCR-UHFFFAOYSA-N
SMILESC[O+](C)C.F[B-](F)(F)F
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream
FormPowder or lumps
Assay (Aqueous acid-base Titration)>95.0%
Solution TestClear (2.5% w/v in water)
Trimethyloxonium tetrafluoroborate act as a avmethylating agent and activates C-X multiple bonds. It is involved in the esterification of polyfunctional carboxylic acids. It acts as a catalyst in the polymerization of cyclic sulfides and ethers. Further, it is used in Beckmann rearrangement of oximes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Trimethyloxonium tetrafluoroborate act as a avmethylating agent and activates C-X multiple bonds. It is involved in the esterification of polyfunctional carboxylic acids. It acts as a catalyst in the polymerization of cyclic sulfides and ethers. Further, it is used in Beckmann rearrangement of oximes.
Solubility
Soluble in nitrobenzene, nitromethane, chloroform, hot acetone, liquid sulfur dioxide. Slightly soluble in dichloromethane. Insoluble in common organic solvents.
Notes
Hygroscopic. Moisture sensitive. Store in a cool place. Incompatible with strong oxidizing agents and bases.
Trimethyloxonium tetrafluoroborate act as a avmethylating agent and activates C-X multiple bonds. It is involved in the esterification of polyfunctional carboxylic acids. It acts as a catalyst in the polymerization of cyclic sulfides and ethers. Further, it is used in Beckmann rearrangement of oximes.
Solubility
Soluble in nitrobenzene, nitromethane, chloroform, hot acetone, liquid sulfur dioxide. Slightly soluble in dichloromethane. Insoluble in common organic solvents.
Notes
Hygroscopic. Moisture sensitive. Store in a cool place. Incompatible with strong oxidizing agents and bases.
RUO – Research Use Only
General References:
- Trialkyloxonium salts, also known as Meerwein's salts, are powerful alkylating agents under mild conditions. For general reactions of trialkyloxonium salts, see Triethyl oxonium tetrafluoroborate, A14420 . For a brief feature of their uses in synthesis, see: Synlett, 195 (2004).
- Alkylation of ethylene thioacetals proceeds only to the monoalkyl salt unless water is added, when the thioacetal is cleaved to give the carbonyl compound and the tetraalkyl ethane bis-sulfonium salt: Synthesis, 135 (1981). Alkylation on S to promote leaving group ability has also been used in a synthesis of oxiranes from ß-hydroxy sulfides: J. Am. Chem. Soc., 95, 3429 (1973); J. Chem. Soc., Chem. Commun., 714 (1975).
- N,N-Dialkyl arylcarboxamides, useful as substrates for directed metallation reactions, can be converted to methyl esters in high yield: Tetrahedron, 56, 9875 (2000).
- Nortcliffe, A.; Moody, C. J. Seven-membered ring scaffolds for drug discovery: Access to functionalised azepanes and oxepanes through diazocarbonyl chemistry. Bioorg. Med. Chem. 2015, 23 (11), 2730-2735.
- Lim, J. Y.; Cunningham, M. J.; Davis, J. J.; Beer, P. D. Halogen bonding-enhanced electrochemical halide anion sensing by redox-active ferrocene receptors. Chem. Commun. 2015, 51 (78), 14640-14643.