Search Thermo Fisher Scientific
Thermo Scientific Chemicals
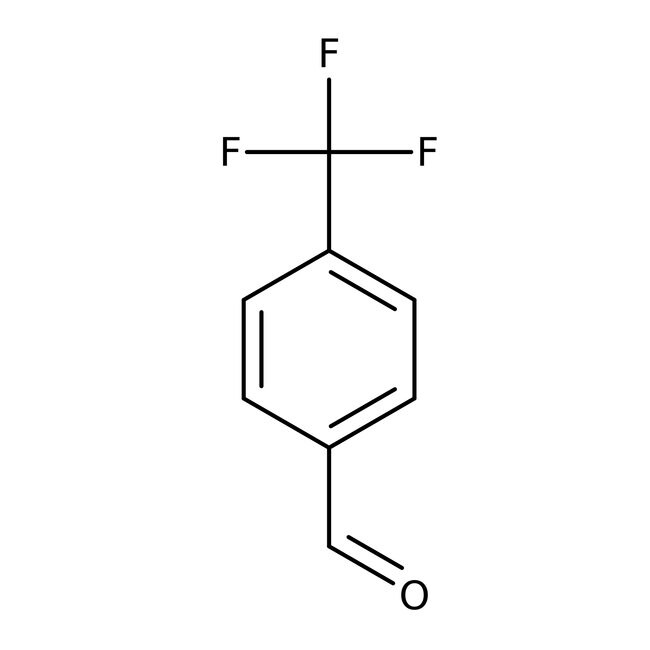
4-(Trifluoromethyl)benzaldehyde, 97%, Thermo Scientific Chemicals
Catalog number A15276.14
also known as A15276-14
Price (USD)/ Each
140.65
Online exclusive
156.00 Save 15.35 (10%)
-
Quantity:
25 g
Price (USD)/ Each
140.65
Online exclusive
156.00 Save 15.35 (10%)
4-(Trifluoromethyl)benzaldehyde, 97%, Thermo Scientific Chemicals
Catalog numberA15276.14
Price (USD)/ Each
140.65
Online exclusive
156.00 Save 15.35 (10%)
-
Chemical Identifiers
CAS455-19-6
IUPAC Name4-(trifluoromethyl)benzaldehyde
Molecular FormulaC8H5F3O
InChI KeyBEOBZEOPTQQELP-UHFFFAOYSA-N
SMILESFC(F)(F)C1=CC=C(C=O)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥96.0%
Appearance (Color)Clear colorless to pale yellow
FormLiquid
CommentMay form precipitate upon standing which does not affect use. Warm gently to redissolve solid.
Free acid (titration)≤1.5%
View more
4-(Trifluoromethyl)benzaldehyde is useful reagent in kinetic studies of the asymmetric synthesis of alcohols and of the Wittig reaction. It is also used as Pharmaceutical intermediates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-(Trifluoromethyl)benzaldehyde is useful reagent in kinetic studies of the asymmetric synthesis of alcohols and of the Wittig reaction. It is also used as Pharmaceutical intermediates.
Solubility
Soluble in water. 1.5 g/L at 20°C
Notes
Air Sensitive. Store away from air and oxidizing agents. Store under an inert atmosphere. Incompatible with oxidizing agents, reducing agents.
4-(Trifluoromethyl)benzaldehyde is useful reagent in kinetic studies of the asymmetric synthesis of alcohols and of the Wittig reaction. It is also used as Pharmaceutical intermediates.
Solubility
Soluble in water. 1.5 g/L at 20°C
Notes
Air Sensitive. Store away from air and oxidizing agents. Store under an inert atmosphere. Incompatible with oxidizing agents, reducing agents.
RUO – Research Use Only
General References:
- Raymond J. Abraham; Simone Angioloni; Mark Edgar and Fernando Sancassan. Conformational analysis. Part 29.1 The conformationalanalysis of 2-substituted fluoro- and trifluoromethyl-benzaldehydes,acetophenones and methyl benzoates by the lanthanide induced shift(LIS)technique.J. Chem. Soc., Perkin Trans. 2.1997, 41-48.
- D C Anderson and F W Dahlquist. Determination of the equilibrium distribution between alcohol and aldehyde substrates when bound to horse liver alcohol dehydrogenase using magnetic resonance.Biochemistry (Washington).1980, 19, (24), 5486-5493 .