Search Thermo Fisher Scientific
Thermo Scientific Chemicals
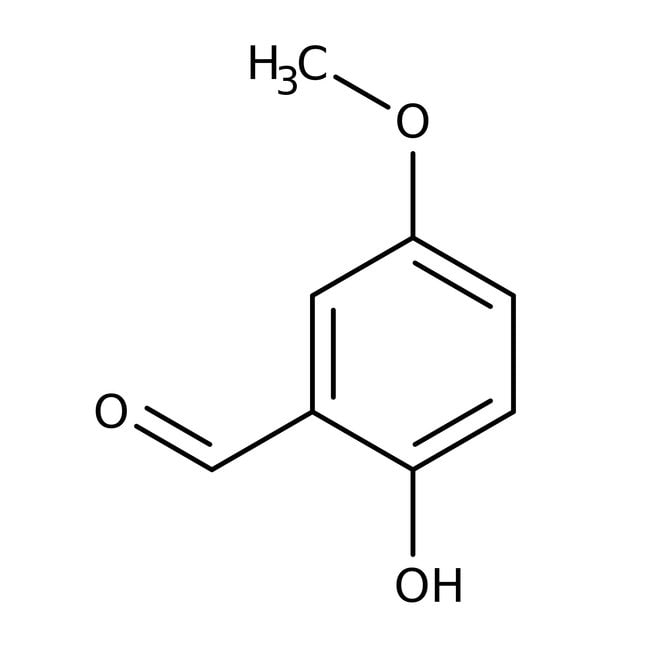
2-Hydroxy-5-methoxybenzaldehyde, 98%, Thermo Scientific Chemicals
Catalog number A15753.14
also known as A15753-14
Price (USD)/ Each
212.65
Online exclusive
236.00 Save 23.35 (10%)
-
Quantity:
25 g
Price (USD)/ Each
212.65
Online exclusive
236.00 Save 23.35 (10%)
2-Hydroxy-5-methoxybenzaldehyde, 98%, Thermo Scientific Chemicals
Catalog numberA15753.14
Price (USD)/ Each
212.65
Online exclusive
236.00 Save 23.35 (10%)
-
Chemical Identifiers
CAS672-13-9
IUPAC Name2-hydroxy-5-methoxybenzaldehyde
Molecular FormulaC8H8O3
InChI KeyFZHSPPYCNDYIKD-UHFFFAOYSA-N
SMILESCOC1=CC=C(O)C(C=O)=C1
View more
Specifications Specification Sheet
Specification Sheet
Formliquid
Appearance (Color)Clear, yellow
Refractive Index1.5770 - 1.5810 @20?C
Assay (GC)> 97.5%
2-Hydroxy-5-methoxybenzaldehyde is employed to study electroantennogram response of the vine weevil (Otiorhynchus sulcatus F) to a broad range of volatile plant compounds. It plays an important role in the preparation of tetradentate Schiff base compounds. Further, it is used in the preparation 6-methoxy-3-nitro-2H-chromene by using dibutylamine as a reagent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Hydroxy-5-methoxybenzaldehyde is employed to study electroantennogram response of the vine weevil (Otiorhynchus sulcatus F) to a broad range of volatile plant compounds. It plays an important role in the preparation of tetradentate Schiff base compounds. Further, it is used in the preparation 6-methoxy-3-nitro-2H-chromene by using dibutylamine as a reagent.
Solubility
Miscible with chloroform. Slightly miscible with water.
Notes
Air sensitive. Incompatible with strong oxidizing agents and strong bases.
2-Hydroxy-5-methoxybenzaldehyde is employed to study electroantennogram response of the vine weevil (Otiorhynchus sulcatus F) to a broad range of volatile plant compounds. It plays an important role in the preparation of tetradentate Schiff base compounds. Further, it is used in the preparation 6-methoxy-3-nitro-2H-chromene by using dibutylamine as a reagent.
Solubility
Miscible with chloroform. Slightly miscible with water.
Notes
Air sensitive. Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Şahin, D.; Koçoğlu, S.; Şener, Ö.; Şenol, C.; Dal, H.; Hökelek, T.; Hayvalı, Z. New NO donor ligands and complexes containing furfuryl or crown ether moiety: Syntheses, crystal structures and tautomerism in ortho-hydroxy substituted compounds as studied by UV-vis spectrophotometry. J. Mol. Struct. 2015, 1102, 302-313.
- Lee, D. S.; Chang, S. M.; Ho, C. Y.; Lu, T. J. Enantioselective Addition of Diethylzinc to Aldehydes Catalyzed by Chiral O,N,O-tridentate Phenol Ligands Derived From Camphor. Chirality 2016, 28 (1), 65-71.