Search Thermo Fisher Scientific
Thermo Scientific Chemicals
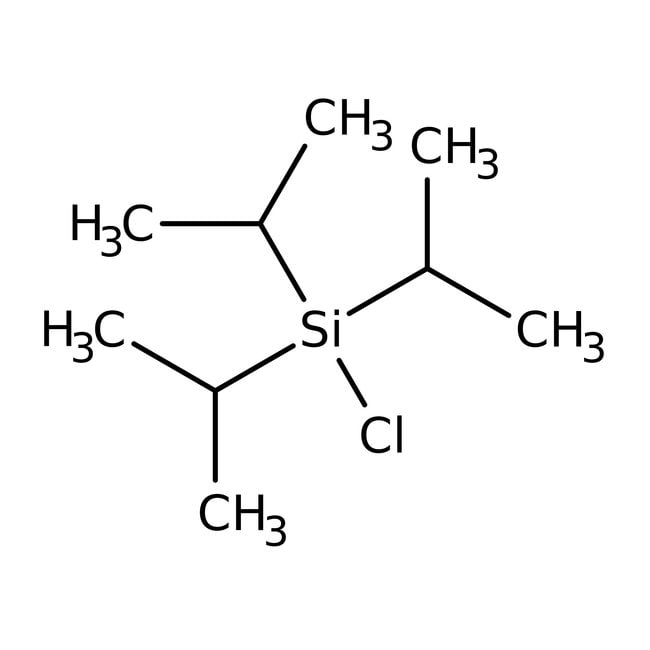
Chlorotriisopropylsilane, 97+%, Thermo Scientific Chemicals
Catalog number A17376.09
also known as A17376-09
Price (USD)/ Each
71.65
Online exclusive
79.80 Save 8.15 (10%)
-
Quantity:
10 g
Price (USD)/ Each
71.65
Online exclusive
79.80 Save 8.15 (10%)
Chlorotriisopropylsilane, 97+%, Thermo Scientific Chemicals
Catalog numberA17376.09
Price (USD)/ Each
71.65
Online exclusive
79.80 Save 8.15 (10%)
-
Chemical Identifiers
CAS13154-24-0
IUPAC Namechlorotris(propan-2-yl)silane
Molecular FormulaC9H21ClSi
InChI KeyKQIADDMXRMTWHZ-UHFFFAOYSA-N
SMILESCC(C)[Si](Cl)(C(C)C)C(C)C
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥97.0%
Appearance (Color)Clear colorless to pale yellow
Refractive Index1.4505-1.4555 @ 20?C
Identification (FTIR)Conforms
FormLiquid
Chlorotriisopropylsilane is used as a silylating agent in neucleotide synthesis. It is used as an intermediate in the manufacture of chemical substances such as pharmaceuticals and in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Chlorotriisopropylsilane is used as a silylating agent in neucleotide synthesis. It is used as an intermediate in the manufacture of chemical substances such as pharmaceuticals and in organic synthesis.
Solubility
Miscible with water.
Notes
Incompatible with strong oxidizing agents, strong bases, alcohols and amines.
Chlorotriisopropylsilane is used as a silylating agent in neucleotide synthesis. It is used as an intermediate in the manufacture of chemical substances such as pharmaceuticals and in organic synthesis.
Solubility
Miscible with water.
Notes
Incompatible with strong oxidizing agents, strong bases, alcohols and amines.
RUO – Research Use Only
General References:
- Chen, Y.; Takada, K.; Kubota, N.; Eric, O.; Ito, T.; Isono, T.; Satoh, T.; Kakuchi, T. Synthesis of end-functionalized poly(methyl methacrylate) by organocatalyzed group transfer polymerization using functional silyl ketene acetals and alpha-phenylacrylates. Poly. Chem. 2015, 6 (10), 1830-1837.
- Tsuya, T.; Iritani, K.; Tahara, K.; Tobe, Y.; Iwanaga, T.; Toyota, S. Chemistry of Anthracene-Acetylene Oligomers XXV: On-Surface Chirality of a Self-Assembled Molecular Network of a Fan-Blade-Shaped Anthracene-Acetylene Macrocycle with a Long Alkyl Chain. Chem. Eur. J. 2015, 21 (14), 5520-5527.